VIAGRA- sildenafil citrate tablet, film coated
Lake Erie Medical DBA Quality Care Products LLC
----------
VIAGRA®
(sildenafil citrate)
Tablets
DESCRIPTION
VIAGRA®, an oral therapy for erectile dysfunction, is the citrate salt of sildenafil, a selective inhibitor of cyclic guanosine monophosphate (cGMP)-specific phosphodiesterase type 5 (PDE5).
Sildenafil citrate is designated chemically as 1-[[3-(6,7-dihydro-1-methyl-7-oxo-3-propyl-1H-pyrazolo[4,3-d]pyrimidin-5-yl)-4-ethoxyphenyl]sulfonyl]-4-methylpiperazine citrate and has the following structural formula:
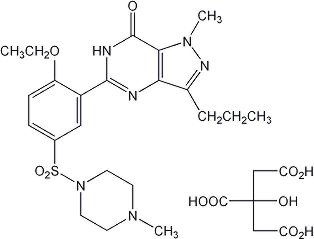
Sildenafil citrate is a white to off-white crystalline powder with a solubility of 3.5 mg/mL in water and a molecular weight of 666.7. VIAGRA (sildenafil citrate) is formulated as blue, film-coated rounded-diamond-shaped tablets equivalent to 25 mg, 50 mg and 100 mg of sildenafil for oral administration. In addition to the active ingredient, sildenafil citrate, each tablet contains the following inactive ingredients: microcrystalline cellulose, anhydrous dibasic calcium phosphate, croscarmellose sodium, magnesium stearate, hypromellose, titanium dioxide, lactose, triacetin, and FD & C Blue #2 aluminum lake.
CONTRAINDICATIONS
Consistent with its known effects on the nitric oxide/cGMP pathway (see CLINICAL PHARMACOLOGY), VIAGRA was shown to potentiate the hypotensive effects of nitrates, and its administration to patients who are using organic nitrates, either regularly and/or intermittently, in any form is therefore contraindicated.
After patients have taken VIAGRA, it is unknown when nitrates, if necessary, can be safely administered. Based on the pharmacokinetic profile of a single 100 mg oral dose given to healthy normal volunteers, the plasma levels of sildenafil at 24 hours post dose are approximately 2 ng/mL (compared to peak plasma levels of approximately 440 ng/mL) (see CLINICAL PHARMACOLOGY: Pharmacokinetics and Metabolism). In the following patients: age >65, hepatic impairment (e.g., cirrhosis), severe renal impairment (e.g., creatinine clearance <30 mL/min), and concomitant use of potent cytochrome P450 3A4 inhibitors (erythromycin), plasma levels of sildenafil at 24 hours post dose have been found to be 3 to 8 times higher than those seen in healthy volunteers. Although plasma levels of sildenafil at 24 hours post dose are much lower than at peak concentration, it is unknown whether nitrates can be safely coadministered at this time point.
VIAGRA is contraindicated in patients with a known hypersensitivity to any component of the tablet.
WARNINGS
There is a potential for cardiac risk of sexual activity in patients with preexisting cardiovascular disease. Therefore, treatments for erectile dysfunction, including VIAGRA, should not be generally used in men for whom sexual activity is inadvisable because of their underlying cardiovascular status.
VIAGRA has systemic vasodilatory properties that resulted in transient decreases in supine blood pressure in healthy volunteers (mean maximum decrease of 8.4/5.5 mmHg), (see CLINICAL PHARMACOLOGY: Pharmacodynamics). While this normally would be expected to be of little consequence in most patients, prior to prescribing VIAGRA, physicians should carefully consider whether their patients with underlying cardiovascular disease could be affected adversely by such vasodilatory effects, especially in combination with sexual activity.
Patients with the following underlying conditions can be particularly sensitive to the actions of vasodilators including VIAGRA – those with left ventricular outflow obstruction (e.g. aortic stenosis, idiopathic hypertrophic subaortic stenosis) and those with severely impaired autonomic control of blood pressure.
There is no controlled clinical data on the safety or efficacy of VIAGRA in the following groups; if prescribed, this should be done with caution.
- Patients who have suffered a myocardial infarction, stroke, or life-threatening arrhythmia within the last 6 months;
- Patients with resting hypotension (BP <90/50) or hypertension (BP >170/110);
- Patients with cardiac failure or coronary artery disease causing unstable angina;
- Patients with retinitis pigmentosa (a minority of these patients have genetic disorders of retinal phosphodiesterases);
- Patients with sickle cell or related anemias.
Prolonged erection greater than 4 hours and priapism (painful erections greater than 6 hours in duration) have been reported infrequently since market approval of VIAGRA. In the event of an erection that persists longer than 4 hours, the patient should seek immediate medical assistance. If priapism is not treated immediately, penile tissue damage and permanent loss of potency could result.
The concomitant administration of the protease inhibitor ritonavir substantially increases serum concentrations of sildenafil (11-fold increase in AUC). If VIAGRA is prescribed to patients taking ritonavir, caution should be used. Data from subjects exposed to high systemic levels of sildenafil are limited. Visual disturbances occurred more commonly at higher levels of sildenafil exposure. Decreased blood pressure, syncope, and prolonged erection were reported in some healthy volunteers exposed to high doses of sildenafil (200–800 mg). To decrease the chance of adverse events in patients taking ritonavir, a decrease in sildenafil dosage is recommended (see Drug Interactions, ADVERSE REACTIONS and DOSAGE AND ADMINISTRATION).
PRECAUTIONS
General
The evaluation of erectile dysfunction should include a determination of potential underlying causes and the identification of appropriate treatment following a complete medical assessment.
Before prescribing VIAGRA, it is important to note the following:
Caution is advised when Phosphodiesterase Type 5 (PDE5) inhibitors are co-administered with alpha-blockers. PDE5 inhibitors, including VIAGRA, and alpha-adrenergic blocking agents are both vasodilators with blood pressure lowering effects. When vasodilators are used in combination, an additive effect on blood pressure may be anticipated. In some patients, concomitant use of these two drug classes can lower blood pressure significantly (see Drug Interactions) leading to symptomatic hypotension (e.g. dizziness, lightheadedness, fainting).
Consideration should be given to the following:
- -
- Patients should be stable on alpha-blocker therapy prior to initiating a PDE5 inhibitor. Patients who demonstrate hemodynamic instability on alpha-blocker therapy alone are at increased risk of symptomatic hypotension with concomitant use of PDE5 inhibitors.
- -
- In those patients who are stable on alpha-blocker therapy, PDE5 inhibitors should be initiated at the lowest dose.
- -
- In those patients already taking an optimized dose of a PDE5 inhibitor, alpha-blocker therapy should be initiated at the lowest dose. Stepwise increase in alpha-blocker dose may be associated with further lowering of blood pressure when taking a PDE5 inhibitor.
- -
- Safety of combined use of PDE5 inhibitors and alpha-blockers may be affected by other variables, including intravascular volume depletion and other anti-hypertensive drugs.
Viagra has systemic vasodilatory properties and may augment the blood pressure lowering effect of other anti-hypertensive medications.
Patients on multiple antihypertensive medications were included in the pivotal clinical trials for VIAGRA. In a separate drug interaction study, when amlodipine, 5 mg or 10 mg, and VIAGRA, 100 mg were orally administered concomitantly to hypertensive patients mean additional blood pressure reduction of 8 mmHg systolic and 7 mmHg diastolic were noted (see Drug Interactions).
The safety of VIAGRA is unknown in patients with bleeding disorders and patients with active peptic ulceration.
VIAGRA should be used with caution in patients with anatomical deformation of the penis (such as angulation, cavernosal fibrosis or Peyronie's disease), or in patients who have conditions which may predispose them to priapism (such as sickle cell anemia, multiple myeloma, or leukemia).
The safety and efficacy of combinations of VIAGRA with other treatments for erectile dysfunction have not been studied. Therefore, the use of such combinations is not recommended.
In humans, VIAGRA has no effect on bleeding time when taken alone or with aspirin. In vitro studies with human platelets indicate that sildenafil potentiates the antiaggregatory effect of sodium nitroprusside (a nitric oxide donor). The combination of heparin and VIAGRA had an additive effect on bleeding time in the anesthetized rabbit, but this interaction has not been studied in humans.
Information for Patients
Physicians should discuss with patients the contraindication of VIAGRA with regular and/or intermittent use of organic nitrates.
Physicians should advise patients of the potential for VIAGRA to augment the blood pressure lowering effect of alpha-blockers and anti-hypertensive medications. Concomitant administration of VIAGRA and an alpha-blocker may lead to symptomatic hypotension in some patients. Therefore, when VIAGRA is co-administered with alpha-blockers, patients should be stable on alpha-blocker therapy prior to initiating VIAGRA treatment and VIAGRA should be initiated at the lowest dose.
Physicians should discuss with patients the potential cardiac risk of sexual activity in patients with preexisting cardiovascular risk factors. Patients who experience symptoms (e.g., angina pectoris, dizziness, nausea) upon initiation of sexual activity should be advised to refrain from further activity and should discuss the episode with their physician.
Physicians should advise patients to stop use of all PDE5 inhibitors, including VIAGRA, and seek medical attention in the event of a sudden loss of vision in one or both eyes. Such an event may be a sign of non-arteritic anterior ischemic optic neuropathy (NAION), a cause of decreased vision including permanent loss of vision, that has been reported rarely post-marketing in temporal association with the use of all PDE5 inhibitors. It is not possible to determine whether these events are related directly to the use of PDE5 inhibitors or to other factors. Physicians should also discuss with patients the increased risk of NAION in individuals who have already experienced NAION in one eye, including whether such individuals could be adversely affected by use of vasodilators, such as PDE5 inhibitors (see POST-MARKETING EXPERIENCE/Special Senses).
Physicians should advise patients to stop taking PDE5 inhibitors, including VIAGRA, and seek prompt medical attention in the event of sudden decrease or loss of hearing. These events, which may be accompanied by tinnitus and dizziness, have been reported in temporal association to the intake of PDE5 inhibitors, including VIAGRA. It is not possible to determine whether these events are related directly to the use of PDE5 inhibitors or to other factors (see ADVERSE REACTIONS, CLINICAL TRIALS and POST-MARKETING EXPERIENCE).
Physicians should warn patients that prolonged erections greater than 4 hours and priapism (painful erections greater than 6 hours in duration) have been reported infrequently since market approval of VIAGRA. In the event of an erection that persists longer than 4 hours, the patient should seek immediate medical assistance. If priapism is not treated immediately, penile tissue damage and permanent loss of potency may result.
Physicians should inform patients not to take VIAGRA with other PDE5 inhibitors including REVATIO. Sildenafil is also marketed as REVATIO for the treatment of pulmonary arterial hypertension. The safety and efficacy of VIAGRA with other PDE5 inhibitors, including REVATIO, have not been studied.
The use of VIAGRA offers no protection against sexually transmitted diseases. Counseling of patients about the protective measures necessary to guard against sexually transmitted diseases, including the Human Immunodeficiency Virus (HIV), may be considered.
Drug Interactions
Effects of Other Drugs on VIAGRA
In vitro studies
Sildenafil metabolism is principally mediated by the cytochrome P450 (CYP) isoforms 3A4 (major route) and 2C9 (minor route). Therefore, inhibitors of these isoenzymes may reduce sildenafil clearance and inducers of these isoenzymes may increase sildenafil clearance.
In vivo studies
Cimetidine (800 mg), a nonspecific CYP inhibitor, caused a 56% increase in plasma sildenafil concentrations when coadministered with VIAGRA (50 mg) to healthy volunteers.
When a single 100 mg dose of VIAGRA was administered with erythromycin, a specific CYP3A4 inhibitor, at steady state (500 mg bid for 5 days), there was a 182% increase in sildenafil systemic exposure (AUC). In addition, in a study performed in healthy male volunteers, coadministration of the HIV protease inhibitor saquinavir, also a CYP3A4 inhibitor, at steady state (1200 mg tid) with VIAGRA (100 mg single dose) resulted in a 140% increase in sildenafil Cmax and a 210% increase in sildenafil AUC. VIAGRA had no effect on saquinavir pharmacokinetics. Stronger CYP3A4 inhibitors such as ketoconazole or itraconazole would be expected to have still greater effects, and population data from patients in clinical trials did indicate a reduction in sildenafil clearance when it was coadministered with CYP3A4 inhibitors (such as ketoconazole, erythromycin, or cimetidine) (see DOSAGE AND ADMINISTRATION).
In another study in healthy male volunteers, coadministration with the HIV protease inhibitor ritonavir, which is a highly potent P450 inhibitor, at steady state (500 mg bid) with VIAGRA (100 mg single dose) resulted in a 300% (4-fold) increase in sildenafil Cmax and a 1000% (11-fold) increase in sildenafil plasma AUC. At 24 hours the plasma levels of sildenafil were still approximately 200 ng/mL, compared to approximately 5 ng/mL when sildenafil was dosed alone. This is consistent with ritonavir's marked effects on a broad range of P450 substrates. VIAGRA had no effect on ritonavir pharmacokinetics (see DOSAGE AND ADMINISTRATION).
Although the interaction between other protease inhibitors and sildenafil has not been studied, their concomitant use is expected to increase sildenafil levels.
In a study of healthy male volunteers, co-administration of sildenafil at steady state (80 mg t.i.d.) with endothelin receptor antagonist bosentan (a moderate inducer of CYP3A4, CYP2C9 and possibly of cytochrome P450 2C19) at steady state (125 mg b.i.d.) resulted in a 63% decrease of sildenafil AUC and a 55% decrease in sildenafil Cmax. Concomitant administration of strong CYP3A4 inducers, such as rifampin, is expected to cause greater decreases in plasma levels of sildenafil.
Single doses of antacid (magnesium hydroxide/aluminum hydroxide) did not affect the bioavailability of VIAGRA.
Pharmacokinetic data from patients in clinical trials showed no effect on sildenafil pharmacokinetics of CYP2C9 inhibitors (such as tolbutamide, warfarin), CYP2D6 inhibitors (such as selective serotonin reuptake inhibitors, tricyclic antidepressants), thiazide and related diuretics, ACE inhibitors, and calcium channel blockers. The AUC of the active metabolite, N-desmethyl sildenafil, was increased 62% by loop and potassium-sparing diuretics and 102% by nonspecific beta-blockers. These effects on the metabolite are not expected to be of clinical consequence.
Effects of VIAGRA on Other Drugs
In vitro studies
Sildenafil is a weak inhibitor of the cytochrome P450 isoforms 1A2, 2C9, 2C19, 2D6, 2E1 and 3A4 (IC50 >150 µM). Given sildenafil peak plasma concentrations of approximately 1 µM after recommended doses, it is unlikely that VIAGRA will alter the clearance of substrates of these isoenzymes.
In vivo studies
Three double-blind, placebo-controlled, randomized, two-way crossover studies were conducted to assess the interaction of VIAGRA with doxazosin, an alpha-adrenergic blocking agent.
In the first study, a single oral dose of VIAGRA 100 mg or matching placebo was administered in a 2-period crossover design to 4 generally healthy males with benign prostatic hyperplasia (BPH). Following at least 14 consecutive daily doses of doxazosin, VIAGRA 100 mg or matching placebo was administered simultaneously with doxazosin. Following a review of the data from these first 4 subjects (details provided below), the VIAGRA dose was reduced to 25 mg. Thereafter, 17 subjects were treated with VIAGRA 25 mg or matching placebo in combination with doxazosin 4 mg (15 subjects) or doxazosin 8mg (2 subjects). The mean subject age was 66.5 years.
For the 17 subjects who received VIAGRA 25 mg and matching placebo, the placebo-subtracted mean maximum decreases from baseline (95% CI) in systolic blood pressure were as follows:
| Placebo-subtracted mean maximum decrease in systolic blood pressure (mm Hg) | VIAGRA 25 mg |
|---|---|
| Supine | 7.4 (-0.9, 15.7) |
| Standing | 6.0 (-0.8, 12.8) |
|
|
| Figure 5: Mean Standing Systolic Blood Pressure Change from Baseline |
Blood pressure was measured immediately pre-dose and at 15, 30, 45 minutes, and 1, 1.5, 2, 2.5, 3, 4, 6 and 8 hours after VIAGRA or matching placebo. Outliers were defined as subjects with a standing systolic blood pressure of <85 mmHg or a decrease from baseline in standing systolic blood pressure of >30 mmHg at one or more timepoints. There were no subjects treated with VIAGRA 25 mg who had a standing SBP < 85mmHg. There were three subjects with a decrease from baseline in standing systolic BP >30mmHg following VIAGRA 25 mg, one subject with a decrease from baseline in standing systolic BP > 30 mmHg following placebo and two subjects with a decrease from baseline in standing systolic BP > 30 mmHg following both VIAGRA and placebo. No severe adverse events potentially related to blood pressure effects were reported in this group.
Of the four subjects who received VIAGRA 100 mg in the first part of this study, a severe adverse event related to blood pressure effect was reported in one patient (postural hypotension that began 35 minutes after dosing with VIAGRA with symptoms lasting for 8 hours), and mild adverse events potentially related to blood pressure effects were reported in two others (dizziness, headache and fatigue at 1 hour after dosing; and dizziness, lightheadedness and nausea at 4 hours after dosing). There were no reports of syncope among these patients. For these four subjects, the placebo-subtracted mean maximum decreases from baseline in supine and standing systolic blood pressures were 14.8 mmHg and 21.5 mmHg, respectively. Two of these subjects had a standing SBP < 85mmHg. Both of these subjects were protocol violators, one due to a low baseline standing SBP, and the other due to baseline orthostatic hypotension.
In the second study, a single oral dose of VIAGRA 50 mg or matching placebo was administered in a 2-period crossover design to 20 generally healthy males with BPH. Following at least 14 consecutive days of doxazosin, VIAGRA 50mg or matching placebo was administered simultaneously with doxazosin 4 mg (17 subjects) or with doxazosin 8 mg (3 subjects). The mean subject age in this study was 63.9 years.
Twenty subjects received VIAGRA 50 mg, but only 19 subjects received matching placebo. One patient discontinued the study prematurely due to an adverse event of hypotension following dosing with VIAGRA 50 mg. This patient had been taking minoxidil, a potent vasodilator, during the study.
For the 19 subjects who received both VIAGRA and matching placebo, the placebo-subtracted mean maximum decreases from baseline (95% CI) in systolic blood pressure were as follows:
| Placebo-subtracted mean maximum decrease in systolic blood pressure (mm Hg) | VIAGRA 50 mg (95% CI) |
|---|---|
| Supine | 9.08 (5.48, 12.68) |
| Standing | 11.62 (7.34, 15.90) |
|
|
| Figure 6: Mean Standing Systolic Blood Pressure Change from Baseline |
Blood pressure was measured after administration of VIAGRA at the same times as those specified for the first doxazosin study. There were two subjects who had a standing SBP of < 85 mmHg. In these two subjects, hypotension was reported as a moderately severe adverse event, beginning at approximately 1 hour after administration of VIAGRA 50 mg and resolving after approximately 7.5 hours. There was one subject with a decrease from baseline in standing systolic BP >30mmHg following VIAGRA 50 mg and one subject with a decrease from baseline in standing systolic BP > 30 mmHg following both VIAGRA 50 mg and placebo. There were no severe adverse events potentially related to blood pressure and no episodes of syncope reported in this study.
In the third study, a single oral dose of VIAGRA 100 mg or matching placebo was administered in a 3-period crossover design to 20 generally healthy males with BPH. In dose period 1, subjects were administered open-label doxazosin and a single dose of VIAGRA 50 mg simultaneously, after at least 14 consecutive days of doxazosin. If a subject did not successfully complete this first dosing period, he was discontinued from the study. Subjects who had successfully completed the previous doxazosin interaction study (using VIAGRA 50 mg), including no significant hemodynamic adverse events, were allowed to skip dose period 1. Treatment with doxazosin continued for at least 7 days after dose period 1. Thereafter, VIAGRA 100mg or matching placebo was administered simultaneously with doxazosin 4 mg (14 subjects) or doxazosin 8 mg (6 subjects) in standard crossover fashion. The mean subject age in this study was 66.4 years.
Twenty-five subjects were screened. Two were discontinued after study period 1: one failed to meet pre-dose screening qualifications and the other experienced symptomatic hypotension as a moderately severe adverse event 30 minutes after dosing with open-label VIAGRA 50 mg. Of the twenty subjects who were ultimately assigned to treatment, a total of 13 subjects successfully completed dose period 1, and seven had successfully completed the previous doxazosin study (using VIAGRA 50 mg).
For the 20 subjects who received VIAGRA 100 mg and matching placebo, the placebo-subtracted mean maximum decreases from baseline (95% CI) in systolic blood pressure were as follows:
| Placebo-subtracted mean maximum decrease in systolic blood pressure (mm Hg) | VIAGRA 100 mg |
|---|---|
| Supine | 7.9 (4.6, 11.1) |
| Standing | 4.3 (-1.8,10.3) |
|
|
| Figure 7: Mean Standing Systolic Blood Pressure Change from Baseline |
Blood pressure was measured after administration of VIAGRA at the same times as those specified for the previous doxazosin studies. There were three subjects who had a standing SBP of < 85 mmHg. All three were taking VIAGRA 100 mg, and all three reported mild adverse events at the time of reductions in standing SBP, including vasodilation and lightheadedness. There were four subjects with a decrease from baseline in standing systolic BP >30mmHg following VIAGRA 100 mg, one subject with a decrease from baseline in standing systolic BP > 30 mmHg following placebo and one subject with a decrease from baseline in standing systolic BP > 30 mmHg following both VIAGRA and placebo. While there were no severe adverse events potentially related to blood pressure reported in this study, one subject reported moderate vasodilatation after both VIAGRA 50 mg and 100 mg. There were no episodes of syncope reported in this study.
When VIAGRA 100 mg oral was coadministered with amlodipine, 5 mg or 10 mg oral, to hypertensive patients, the mean additional reduction on supine blood pressure was 8 mmHg systolic and 7 mmHg diastolic.
No significant interactions were shown with tolbutamide (250 mg) or warfarin (40 mg), both of which are metabolized by CYP2C9.
VIAGRA (50 mg) did not potentiate the increase in bleeding time caused by aspirin (150 mg).
VIAGRA (50 mg) did not potentiate the hypotensive effect of alcohol in healthy volunteers with mean maximum blood alcohol levels of 0.08%.
In a study of healthy male volunteers, sildenafil (100 mg) did not affect the steady state pharmacokinetics of the HIV protease inhibitors, saquinavir and ritonavir, both of which are CYP3A4 substrates.
Sildenafil at steady state (80 mg t.i.d.) resulted in a 50% increase in AUC and a 42% increase in Cmax of bosentan (125 mg b.i.d.).
Carcinogenesis, Mutagenesis, Impairment of Fertility
Sildenafil was not carcinogenic when administered to rats for 24 months at a dose resulting in total systemic drug exposure (AUCs) for unbound sildenafil and its major metabolite of 29- and 42-times, for male and female rats, respectively, the exposures observed in human males given the Maximum Recommended Human Dose (MRHD) of 100 mg. Sildenafil was not carcinogenic when administered to mice for 18–21 months at dosages up to the Maximum Tolerated Dose (MTD) of 10 mg/kg/day, approximately 0.6 times the MRHD on a mg/m2 basis.
Sildenafil was negative in in vitro bacterial and Chinese hamster ovary cell assays to detect mutagenicity, and in vitro human lymphocytes and in vivo mouse micronucleus assays to detect clastogenicity.
There was no impairment of fertility in rats given sildenafil up to 60 mg/kg/day for 36 days to females and 102 days to males, a dose producing an AUC value of more than 25 times the human male AUC.
There was no effect on sperm motility or morphology after single 100 mg oral doses of VIAGRA in healthy volunteers.
Pregnancy, Nursing Mothers and Pediatric Use
VIAGRA is not indicated for use in newborns, children, or women.
Pregnancy Category B
No evidence of teratogenicity, embryotoxicity or fetotoxicity was observed in rats and rabbits which received up to 200 mg/kg/day during organogenesis. These doses represent, respectively, about 20 and 40 times the MRHD on a mg/m2 basis in a 50 kg subject. In the rat pre- and postnatal development study, the no observed adverse effect dose was 30 mg/kg/day given for 36 days. In the nonpregnant rat the AUC at this dose was about 20 times human AUC. There are no adequate and well-controlled studies of sildenafil in pregnant women.
Geriatric Use
Healthy elderly volunteers (65 years or over) had a reduced clearance of sildenafil (see CLINICAL PHARMACOLOGY: Pharmacokinetics in Special Populations). Since higher plasma levels may increase both the efficacy and incidence of adverse events, a starting dose of 25 mg should be considered (see DOSAGE AND ADMINISTRATION).
ADVERSE REACTIONS
CLINICAL TRIALS
VIAGRA was administered to over 3700 patients (aged 19–87 years) during pre-marketing clinical trials worldwide. Over 550 patients were treated for longer than one year.
In placebo-controlled clinical studies, the discontinuation rate due to adverse events for VIAGRA (2.5%) was not significantly different from placebo (2.3%). The adverse events were generally transient and mild to moderate in nature.
In trials of all designs, adverse events reported by patients receiving VIAGRA were generally similar. In fixed-dose studies, the incidence of some adverse events increased with dose. The nature of the adverse events in flexible-dose studies, which more closely reflect the recommended dosage regimen, was similar to that for fixed-dose studies.
When VIAGRA was taken as recommended (on an as-needed basis) in flexible-dose, placebo-controlled clinical trials, the following adverse events were reported:
| Adverse Event | Percentage of Patients Reporting Event | |
|---|---|---|
| VIAGRA N=734 | PLACEBO N=725 |
|
|
||
| Headache | 16% | 4% |
| Flushing | 10% | 1% |
| Dyspepsia | 7% | 2% |
| Nasal Congestion | 4% | 2% |
| Urinary Tract Infection | 3% | 2% |
| Abnormal Vision* | 3% | 0% |
| Diarrhea | 3% | 1% |
| Dizziness | 2% | 1% |
| Rash | 2% | 1% |
Other adverse reactions occurred at a rate of >2%, but equally common on placebo: respiratory tract infection, back pain, flu syndrome, and arthralgia.
In fixed-dose studies, dyspepsia (17%) and abnormal vision (11%) were more common at 100 mg than at lower doses. At doses above the recommended dose range, adverse events were similar to those detailed above but generally were reported more frequently.
The following events occurred in <2% of patients in controlled clinical trials; a causal relationship to VIAGRA is uncertain. Reported events include those with a plausible relation to drug use; omitted are minor events and reports too imprecise to be meaningful:
Body as a whole: face edema, photosensitivity reaction, shock, asthenia, pain, chills, accidental fall, abdominal pain, allergic reaction, chest pain, accidental injury.
Cardiovascular: angina pectoris, AV block, migraine, syncope, tachycardia, palpitation, hypotension, postural hypotension, myocardial ischemia, cerebral thrombosis, cardiac arrest, heart failure, abnormal electrocardiogram, cardiomyopathy.
Digestive: vomiting, glossitis, colitis, dysphagia, gastritis, gastroenteritis, esophagitis, stomatitis, dry mouth, liver function tests abnormal, rectal hemorrhage, gingivitis.
Hemic and Lymphatic: anemia and leukopenia.
Metabolic and Nutritional: thirst, edema, gout, unstable diabetes, hyperglycemia, peripheral edema, hyperuricemia, hypoglycemic reaction, hypernatremia.
Musculoskeletal: arthritis, arthrosis, myalgia, tendon rupture, tenosynovitis, bone pain, myasthenia, synovitis.
Nervous: ataxia, hypertonia, neuralgia, neuropathy, paresthesia, tremor, vertigo, depression, insomnia, somnolence, abnormal dreams, reflexes decreased, hypesthesia.
Respiratory: asthma, dyspnea, laryngitis, pharyngitis, sinusitis, bronchitis, sputum increased, cough increased.
Skin and Appendages: urticaria, herpes simplex, pruritus, sweating, skin ulcer, contact dermatitis, exfoliative dermatitis.
Special Senses: sudden decrease or loss of hearing, mydriasis, conjunctivitis, photophobia, tinnitus, eye pain, ear pain, eye hemorrhage, cataract, dry eyes.
Urogenital: cystitis, nocturia, urinary frequency, breast enlargement, urinary incontinence, abnormal ejaculation, genital edema and anorgasmia.
POST-MARKETING EXPERIENCE
Cardiovascular and cerebrovascular
Serious cardiovascular, cerebrovascular, and vascular events, including myocardial infarction, sudden cardiac death, ventricular arrhythmia, cerebrovascular hemorrhage, transient ischemic attack, hypertension, subarachnoid and intracerebral hemorrhages, and pulmonary hemorrhage have been reported post-marketing in temporal association with the use of VIAGRA. Most, but not all, of these patients had preexisting cardiovascular risk factors. Many of these events were reported to occur during or shortly after sexual activity, and a few were reported to occur shortly after the use of VIAGRA without sexual activity. Others were reported to have occurred hours to days after the use of VIAGRA and sexual activity. It is not possible to determine whether these events are related directly to VIAGRA, to sexual activity, to the patient's underlying cardiovascular disease, to a combination of these factors, or to other factors (see WARNINGS for further important cardiovascular information).
Special senses
Cases of sudden decrease or loss of hearing have been reported postmarketing in temporal association with the use of PDE5 inhibitors, including VIAGRA. In some of the cases, medical conditions and other factors were reported that may have also played a role in the otologic adverse events. In many cases, medical follow-up information was limited. It is not possible to determine whether these reported events are related directly to the use of VIAGRA, to the patient's underlying risk factors for hearing loss, a combination of these factors, or to other factors (see PRECAUTIONS, Information for Patients).
Other events
Other events reported post-marketing to have been observed in temporal association with VIAGRA and not listed in the clinical trial adverse reactions section above include:
Nervous: seizure, seizure recurrence, anxiety, and transient global amnesia.
Urogenital: prolonged erection, priapism (see WARNINGS), and hematuria.
Special Senses: diplopia, temporary vision loss/decreased vision, ocular redness or bloodshot appearance, ocular burning, ocular swelling/pressure, increased intraocular pressure, retinal vascular disease or bleeding, vitreous detachment/traction, paramacular edema and epistaxis.
Non-arteritic anterior ischemic optic neuropathy (NAION), a cause of decreased vision including permanent loss of vision, has been reported rarely post-marketing in temporal association with the use of phosphodiesterase type 5 (PDE5) inhibitors, including VIAGRA. Most, but not all, of these patients had underlying anatomic or vascular risk factors for developing NAION, including but not necessarily limited to: low cup to disc ratio ("crowded disc"), age over 50, diabetes, hypertension, coronary artery disease, hyperlipidemia and smoking. It is not possible to determine whether these events are related directly to the use of PDE5 inhibitors, to the patient's underlying vascular risk factors or anatomical defects, to a combination of these factors, or to other factors (see PRECAUTIONS/Information for Patients).
Hemic and Lymphatic: Vaso-occlusive crisis: In a small, prematurely terminated study of REVATIO in patients with pulmonary hypertension (PH) secondary to sickle cell disease, vaso-occlusive crises requiring hospitalization were more commonly reported in patients who received sildenafil than in those randomized to placebo. The clinical relevance of this finding to men treated with VIAGRA for ED is not known.
OVERDOSAGE
In studies with healthy volunteers of single doses up to 800 mg, adverse events were similar to those seen at lower doses but incidence rates and severities were increased.
In cases of overdose, standard supportive measures should be adopted as required. Renal dialysis is not expected to accelerate clearance as sildenafil is highly bound to plasma proteins and it is not eliminated in the urine.
DOSAGE AND ADMINISTRATION
For most patients, the recommended dose is 50 mg taken, as needed, approximately 1 hour before sexual activity. However, VIAGRA may be taken anywhere from 4 hours to 0.5 hour before sexual activity. Based on effectiveness and toleration, the dose may be increased to a maximum recommended dose of 100 mg or decreased to 25 mg. The maximum recommended dosing frequency is once per day.
The following factors are associated with increased plasma levels of sildenafil: age >65 (40% increase in AUC), hepatic impairment (e.g., cirrhosis, 80%), severe renal impairment (creatinine clearance <30 mL/min, 100%), and concomitant use of potent cytochrome P450 3A4 inhibitors [ketoconazole, itraconazole, erythromycin (182%), saquinavir (210%)]. Since higher plasma levels may increase both the efficacy and incidence of adverse events, a starting dose of 25 mg should be considered in these patients.
Ritonavir greatly increased the systemic level of sildenafil in a study of healthy, non-HIV infected volunteers (11-fold increase in AUC, see Drug Interactions.) Based on these pharmacokinetic data, it is recommended not to exceed a maximum single dose of 25 mg of VIAGRA in a 48 hour period.
VIAGRA was shown to potentiate the hypotensive effects of nitrates and its administration in patients who use nitric oxide donors or nitrates in any form is therefore contraindicated.
When VIAGRA is co-administered with an alpha-blocker, patients should be stable on alpha-blocker therapy prior to initiating VIAGRA treatment and VIAGRA should be initiated at the lowest dose (see Drug Interactions).
HOW SUPPLIED
VIAGRA (sildenafil citrate) is supplied as blue, film-coated, rounded-diamond-shaped tablets containing sildenafil citrate equivalent to the nominally indicated amount of sildenafil as follows:
| 25 mg | 50 mg | 100 mg | |
|---|---|---|---|
| Obverse | VGR25 | VGR50 | VGR100 |
| Reverse | PFIZER | PFIZER | PFIZER |
| Bottle of 30 | NDC-0069-4200-30 | NDC-0069-4210-30 | NDC-0069-4220-30 |
| Bottle of 100 | N/A | NDC-0069-4210-66 | NDC-0069-4220-66 |
PATIENT SUMMARY OF INFORMATION ABOUT
This summary contains important information about VIAGRA®. It is not meant to take the place of your doctor's instructions. Read this information carefully before you start taking VIAGRA. Ask your doctor or pharmacist if you do not understand any of this information or if you want to know more about VIAGRA.
This medicine can help many men when it is used as prescribed by their doctors. However, VIAGRA is not for everyone. It is intended for use only by men who have a condition called erectile dysfunction. VIAGRA must never be used by men who are taking medicines that contain nitrates of any kind, at any time. This includes nitroglycerin. If you take VIAGRA with any nitrate medicine your blood pressure could suddenly drop to an unsafe or life threatening level.
• WHAT IS VIAGRA?
VIAGRA is a pill used to treat erectile dysfunction (impotence) in men. It can help many men who have erectile dysfunction get and keep an erection when they become sexually excited (stimulated).
You will not get an erection just by taking this medicine. VIAGRA helps a man with erectile dysfunction get an erection only when he is sexually excited.
• HOW SEX AFFECTS THE BODY
When a man is sexually excited, the penis rapidly fills with more blood than usual. The penis then expands and hardens. This is called an erection. After the man is done having sex, this extra blood flows out of the penis back into the body. The erection goes away. If an erection lasts for a long time (more than 6 hours), it can permanently damage your penis. You should call a doctor immediately if you ever have a prolonged erection that lasts more than 4 hours.
Some conditions and medicines interfere with this natural erection process. The penis cannot fill with enough blood. The man cannot have an erection. This is called erectile dysfunction if it becomes a frequent problem.
During sex, your heart works harder. Therefore sexual activity may not be advisable for people who have heart problems. Before you start any treatment for erectile dysfunction, ask your doctor if your heart is healthy enough to handle the extra strain of having sex. If you have chest pains, dizziness or nausea during sex, stop having sex and immediately tell your doctor you have had this problem.
• HOW VIAGRA WORKS
VIAGRA enables many men with erectile dysfunction to respond to sexual stimulation. When a man is sexually excited, VIAGRA helps the penis fill with enough blood to cause an erection. After sex is over, the erection goes away.
• VIAGRA IS NOT FOR EVERYONE
As noted above (How Sex Affects the Body), ask your doctor if your heart is healthy enough for sexual activity.
If you take any medicines that contain nitrates – either regularly or as needed – you should never take VIAGRA. If you take VIAGRA with any nitrate medicine or recreational drug containing nitrates, your blood pressure could suddenly drop to an unsafe level. You could get dizzy, faint, or even have a heart attack or stroke. Nitrates are found in many prescription medicines that are used to treat angina (chest pain due to heart disease) such as:
- nitroglycerin (sprays, ointments, skin patches or pastes, and tablets that are swallowed or dissolved in the mouth)
- isosorbide mononitrate and isosorbide dinitrate (tablets that are swallowed, chewed, or dissolved in the mouth)
Nitrates are also found in recreational drugs such as amyl nitrate or nitrite ("poppers"). If you are not sure if any of your medicines contain nitrates, or if you do not understand what nitrates are, ask your doctor or pharmacist.
VIAGRA is only for patients with erectile dysfunction. VIAGRA is not for newborns, children, or women. Do not let anyone else take your VIAGRA. VIAGRA must be used only under a doctor's supervision.
• WHAT VIAGRA DOES NOT DO
- VIAGRA does not cure erectile dysfunction. It is a treatment for erectile dysfunction.
- VIAGRA does not protect you or your partner from getting sexually transmitted diseases, including HIV—the virus that causes AIDS.
- VIAGRA is not a hormone or an aphrodisiac.
• WHAT TO TELL YOUR DOCTOR BEFORE YOU BEGIN VIAGRA
Only your doctor can decide if VIAGRA is right for you. VIAGRA can cause mild, temporary lowering of your blood pressure. You will need to have a thorough medical exam to diagnose your erectile dysfunction and to find out if you can safely take VIAGRA alone or with your other medicines. Your doctor should determine if your heart is healthy enough to handle the extra strain of having sex.
Be sure to tell your doctor if you:
- have ever had any heart problems (e.g., angina, chest pain, heart failure, irregular heart beats, heart attack or narrowing of the aortic valve)
- have ever had a stroke
- have low or high blood pressure
- have ever had severe vision loss
- have a rare inherited eye disease called retinitis pigmentosa
- have ever had any kidney problems
- have ever had any liver problems
- have ever had any blood problems, including sickle cell anemia or leukemia
- are allergic to sildenafil or any of the other ingredients of VIAGRA tablets
- have a deformed penis, Peyronie's disease, or ever had an erection that lasted more than 4 hours
- have stomach ulcers or any types of bleeding problems
- are taking any other medicines
• VIAGRA AND OTHER MEDICINES
Some medicines can change the way VIAGRA works. Tell your doctor about any medicines you are taking. Do not start or stop taking any medicines before checking with your doctor or pharmacist. This includes prescription and nonprescription medicines or remedies:
- Remember, VIAGRA should never be used with medicines that contain nitrates (see VIAGRA Is Not for Everyone).
- If you are taking medicines called alpha-blockers for the treatment of high blood pressure or prostate problems, your blood pressure could suddenly drop. You could get dizzy or faint.
- If you are taking a protease inhibitor, your dose may be adjusted (please see Finding the Right Dose for You).
- VIAGRA should not be used with any other medical treatments that cause erections. These treatments include pills, medicines that are injected or inserted into the penis, implants or vacuum pumps.
- VIAGRA contains sildenafil, which is the same medicine found in another drug called REVATIO. REVATIO is used to treat a rare disease called pulmonary arterial hypertension. VIAGRA should not be used with REVATIO.
• FINDING THE RIGHT DOSE FOR YOU
VIAGRA comes in different doses (25 mg, 50 mg and 100 mg). If you do not get the results you expect, talk with your doctor. You and your doctor can determine the dose that works best for you.
- Do not take more VIAGRA than your doctor prescribes.
- If you think you need a larger dose of VIAGRA, check with your doctor.
- VIAGRA should not be taken more than once a day.
Your doctor may prescribe a lower dose of VIAGRA in certain circumstances. For example:
- If you are older than age 65, or have serious liver or kidney problems, your doctor may start you at the lowest dose (25 mg) of VIAGRA.
- If you are taking protease inhibitors, such as for the treatment of HIV, your doctor may recommend a 25 mg dose and may limit you to a maximum single dose of 25 mg of VIAGRA in a 48 hour period.
- If you have prostate problems or high blood pressure for which you take medicines called alpha blockers, your doctor may start you on a lower dose of VIAGRA.
• HOW TO TAKE VIAGRA
Take VIAGRA about one hour before you plan to have sex. Beginning in about 30 minutes and for up to 4 hours, VIAGRA can help you get an erection if you are sexually excited. If you take VIAGRA after a high-fat meal (such as a cheeseburger and french fries), the medicine may take a little longer to start working. VIAGRA can help you get an erection when you are sexually excited. You will not get an erection just by taking the pill.
• POSSIBLE SIDE EFFECTS
Like all medicines, VIAGRA can cause some side effects. These effects are usually mild to moderate and usually don't last longer than a few hours. Some of these side effects are more likely to occur with higher doses. The most common side effects of VIAGRA are headache, flushing of the face, and upset stomach. Less common side effects that may occur are temporary changes in color vision (such as trouble telling the difference between blue and green objects or having a blue color tinge to them), eyes being more sensitive to light, or blurred vision.
In rare instances, men taking PDE5 inhibitors (oral erectile dysfunction medicines, including VIAGRA) reported a sudden decrease or loss of vision in one or both eyes. It is not possible to determine whether these events are related directly to these medicines, to other factors such as high blood pressure or diabetes, or to a combination of these. If you experience sudden decrease or loss of vision, stop taking PDE5 inhibitors, including VIAGRA, and call a doctor right away.
In rare instances, men have reported an erection that lasts many hours. You should call a doctor immediately if you ever have an erection that lasts more than 4 hours. If not treated right away, permanent damage to your penis could occur (see How Sex Affects the Body).
Sudden loss or decrease in hearing, sometimes with ringing in the ears and dizziness, has been rarely reported in people taking PDE5 inhibitors, including VIAGRA. It is not possible to determine whether these events are related directly to the PDE5 inhibitors, to other diseases or medications, to other factors, or to a combination of factors. If you experience these symptoms, stop taking VIAGRA and contact a doctor right away.
Heart attack, stroke, irregular heart beats, and death have been reported rarely in men taking VIAGRA. Most, but not all, of these men had heart problems before taking this medicine. It is not possible to determine whether these events were directly related to VIAGRA.
VIAGRA may cause other side effects besides those listed on this sheet. If you want more information or develop any side effects or symptoms you are concerned about, call your doctor.
• ACCIDENTAL OVERDOSE
In case of accidental overdose, call your doctor right away.
• STORING VIAGRA
Keep VIAGRA out of the reach of children. Keep VIAGRA in its original container. Store at 25°C (77°F); excursions permitted to 15–30°C (59–86°F) [see USP Controlled Room Temperature].
• FOR MORE INFORMATION ON VIAGRA
VIAGRA is a prescription medicine used to treat erectile dysfunction. Only your doctor can decide if it is right for you. This sheet is only a summary. If you have any questions or want more information about VIAGRA, talk with your doctor or pharmacist, visit www.viagra.com, or call 1-888-4VIAGRA.

LAB-0220-7.0
January 2010
| VIAGRA
sildenafil citrate tablet, film coated |
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Labeler - Lake Erie Medical DBA Quality Care Products LLC (831276758) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Lake Erie Medical DBA Quality Care Products LLC | 831276758 | repack(49999-316) | |

