SUCRETS BLACK CHERRY- dyclonine hydrochloride lozenge
Insight Pharmaceuticals LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
SUCRETS®
COMPLETE
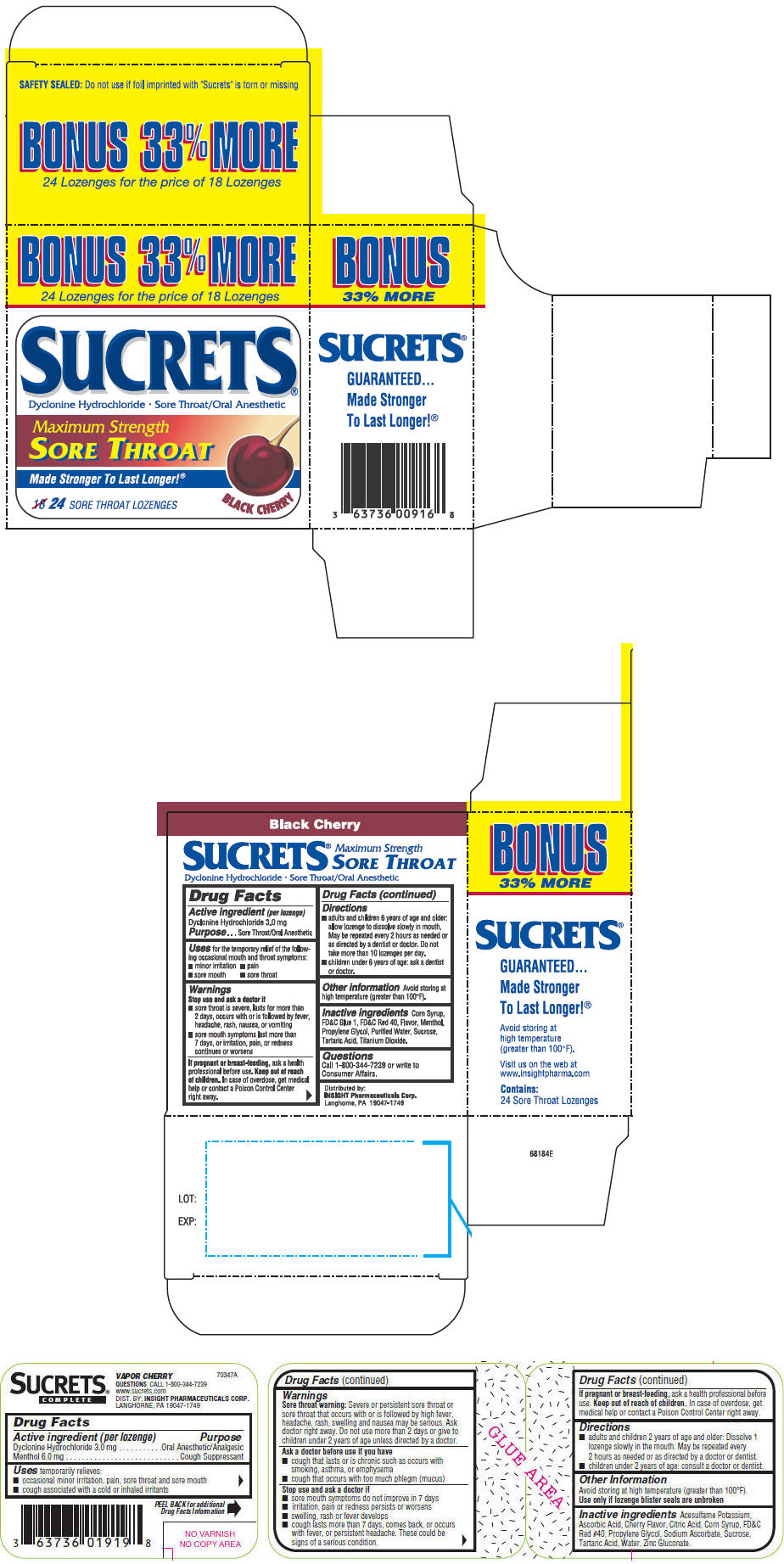
BLACK CHERRY
Uses
temporarily relieves:
- occasional minor irritation, pain, sore throat and sore mouth
- cough associated with a cold or inhaled irritants
Warnings
Sore throat warning
Severe or persistent sore throat or sore throat that occurs with or is followed by high fever, headache, rash, swelling and nausea may be serious. Ask doctor right away. Do not use more than 2 days or give to children under 2 years of age unless directed by a doctor.
Ask a doctor before use if you have
- cough that lasts or is chronic such as occurs with smoking, asthma, or emphysema
- cough that occurs with too much phlegm (mucus)
Directions
- adults and children 2 years of age and older: Dissolve 1 lozenge slowly in the mouth. May be repeated every 2 hours as needed or as directed by a doctor or dentist.
- children under 2 years of age: consult a doctor or dentist.
Other Information
Avoid storing at high temperature (greater than 100°F).
Use only if lozenge blister seals are unbroken
| SUCRETS
BLACK CHERRY
dyclonine hydrochloride lozenge |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| Labeler - Insight Pharmaceuticals LLC (055665422) |
Revised: 11/2011
Document Id: ec060aae-ffaf-48ba-a00f-c6afc6ceb96a
Set id: 3ffdf9c8-26a0-4373-8e15-c4fbbad79efe
Version: 4
Effective Time: 20111101
Insight Pharmaceuticals LLC