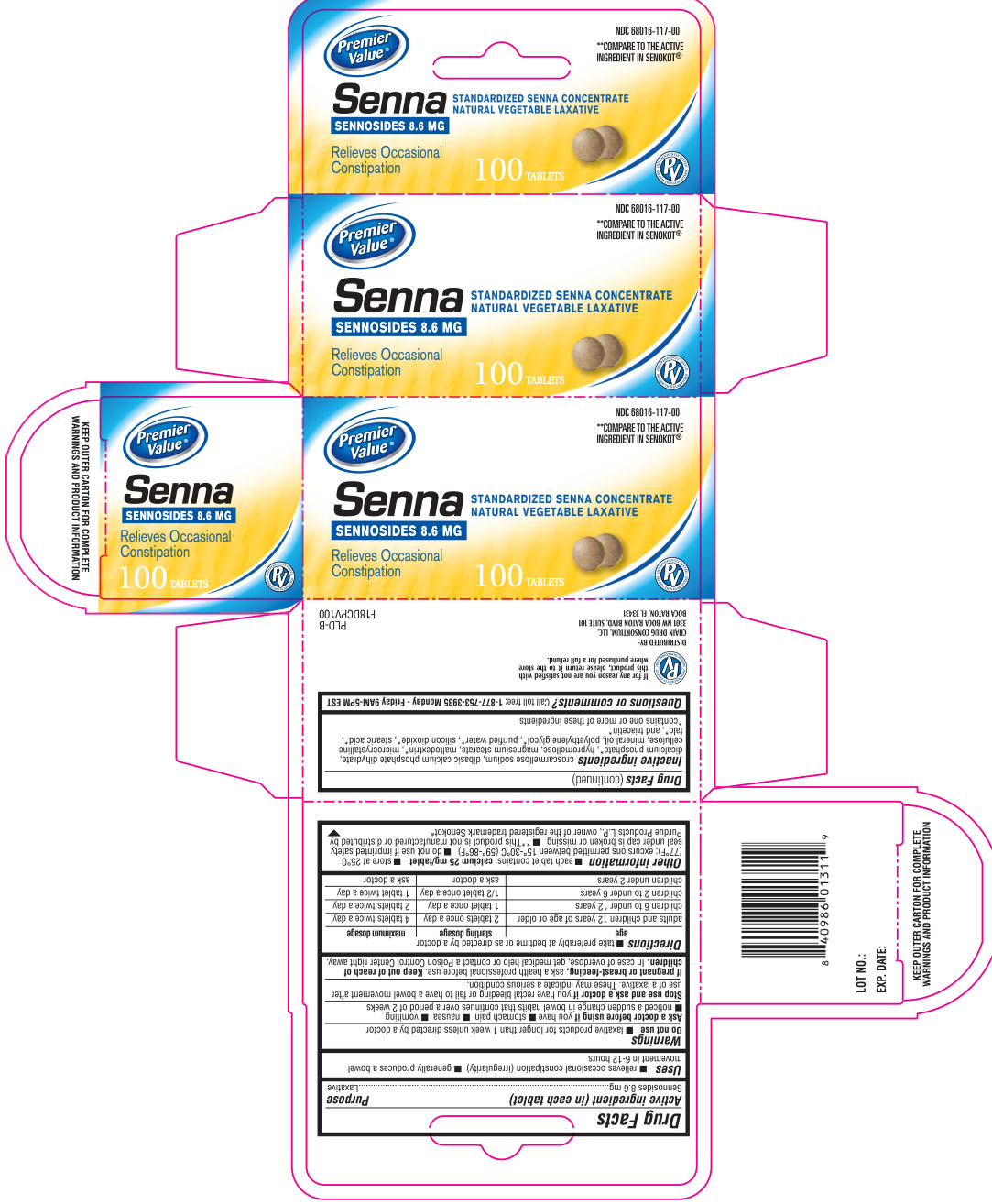
SENNA- sennosides tablet
Chain Drug Consortium, LLC
----------
DRUG FACTS
Uses
- relieves occasional constipation (irregularity)
- generally produces a bowel movement within 6- 12 hours.
Warnings
Do not use
- laxative products for longer than 1 week unless directed by a doctor.
Ask a doctor before using if
you have
- stomach pain
- nausea
- vomiting
- noticed a sudden change in bowel movements that continues over a period of 2 weeks.
Directions
- Take preferably at bedtime or as directed by a doctor
|
age | starting dosage | maximum dosage |
| adults and children 12 years of age or older | 2 tablets once a day | 4 tablets twice a day |
| children 6 to under 12 years | 1 tablet once a day | 2 tablets twice a day |
| children 2 to under 6 years | 1/2 tablet once a day | 1 tablet twice a day |
| children under 2 years | ask a doctor | ask a doctor |
Other information
- each tablet contains: calcium 25 mg/ tablet
- store at 25o C (77o F); excursions permitted between 15o - 30o C (59o-86o F)
- do not use if imprinted safety seal under cap is broken or missing.
- **This product is not manufactured or distributed by Purdue Products, LP, owner of the registered trademark Senokot®
Inactive Ingredients
croscarmellose sodium, dibasic calcium phosphate dihydrate, dicalcium phosphate*, hypromellose, magnesium stearate, maltodextrin*, microcrystalline cellulose, mineral oil, polyethylene glycol*, purified water*, silicon dioxide*, stearic acid*, talc*, and triacetin*
*contains one or more of these ingredients
Principal Display Panel
**Compare to the active ingredient in senokot®
Senna
Sennosides 8.6 mg
Standardized senna concentrate
Natural vegetable laxative
Relieves occasional constipation
Distributed by:
CHAIN DRUG CONSORTIUM, LLC
3301 NW BOCA RATON BLVD. SUITE 101
BOCA RATON, FL 33431
KEEP OUTER CARTON FOR COMPLETE WARNINGS AND PRODUCT INFORMATION
| SENNA
sennosides tablet |
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
| Labeler - Chain Drug Consortium, LLC (101668460) |
Revised: 3/2024
Document Id: 65276b5e-2400-45dd-b56e-989f28239bd5
Set id: 3f43ff4b-c6e5-42a6-b2da-dc5e862f2c76
Version: 5
Effective Time: 20240306
Chain Drug Consortium, LLC