Label: NASALASALT- sodium chloride spray
-
Contains inactivated NDC Code(s)
NDC Code(s): 52429-123-01 - Packager: Nasal and Sinus Health, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated September 26, 2011
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- USES
-
DIRECTIONS
Tilt head forward, spray 2-3 times in each nostril directing spray upward and outward toward ear and away from center of nose. Blow nose. For best results, see package insert for the Vanderpool Technique™ for a more thorough nasal cleansing procedure or go to the NasalAsalt® web site for a video demonstration.
- WARNINGS
- PRECAUTIONS
- ACTIVE INGREDIENT
- SPL UNCLASSIFIED SECTION
-
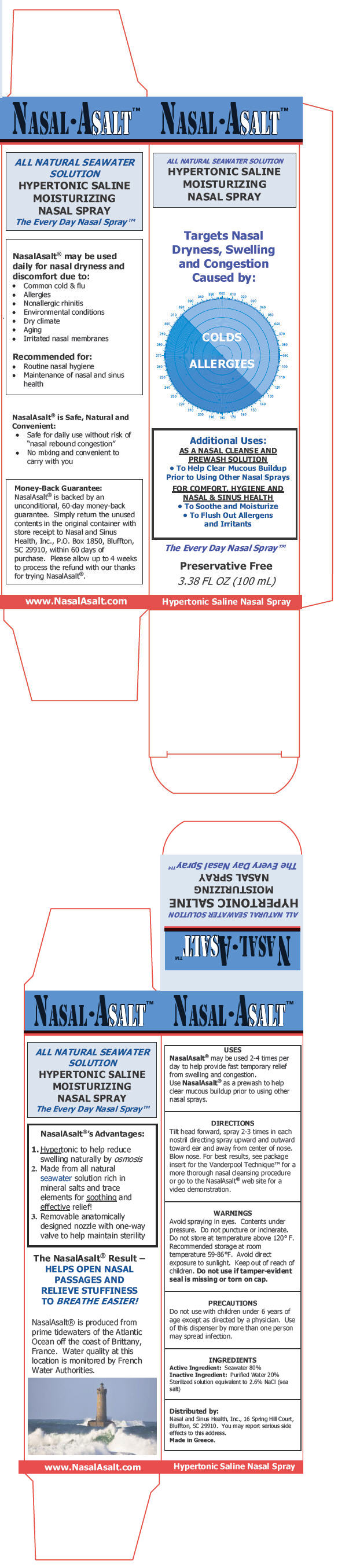
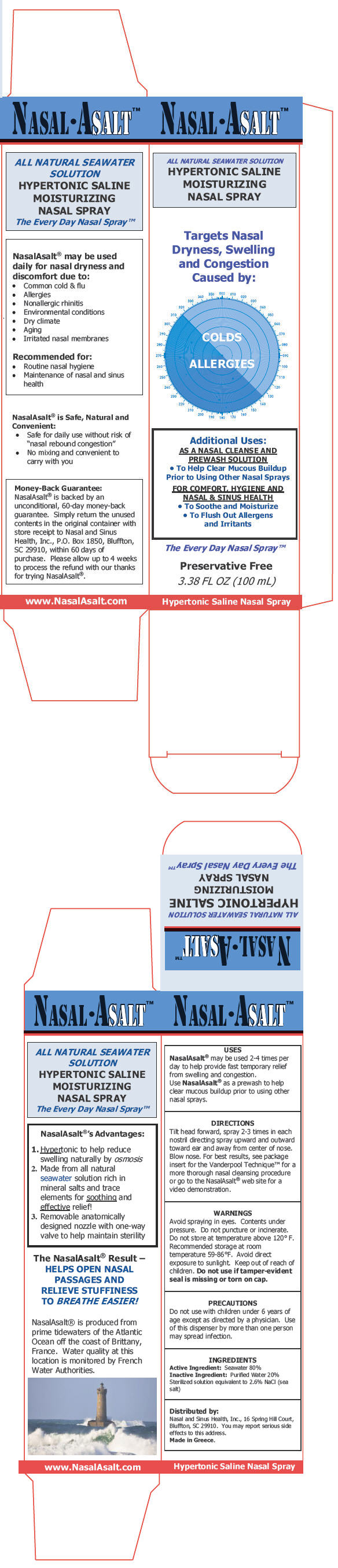
PRINCIPAL DISPLAY PANEL - 100 mL Canister Carton
NASAL•ASALT™
ALL NATURAL SEAWATER SOLUTION
HYPERTONIC SALINE
MOISTURIZING
NASAL SPRAYTargets Nasal
Dryness, Swelling
and Congestion
Caused by:COLDS
ALLERGIESAdditional Uses:
AS A NASAL CLEANSE AND
PREWASH SOLUTION-
To Help Clear Mucous Buildup
Prior to Using Other Nasal Sprays
FOR COMFORT, HYGIENE AND
NASAL & SINUS HEALTH- To Soothe and Moisturize
-
To Flush Out Allergens
and Irritants
The Every Day Nasal Spray™
Preservative Free
3.38 FL OZ (100 mL)
Hypertonic Saline Nasal Spray
-
To Help Clear Mucous Buildup
-
INGREDIENTS AND APPEARANCE
NASALASALT ALL-NATURAL SEAWATER
sodium chloride sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:52429-123 Route of Administration NASAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Sodium Chloride (UNII: 451W47IQ8X) (Sodium Cation - UNII:LYR4M0NH37) Sodium Chloride 2600 mg in 100 mL Inactive Ingredients Ingredient Name Strength water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:52429-123-01 1 in 1 BOX 1 100 mL in 1 CANISTER Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part349 11/01/2011 Labeler - Nasal and Sinus Health, Inc. (968415740) Establishment Name Address ID/FEI Business Operations Nasal and Sinus Health, Inc. 968415740 RELABEL Establishment Name Address ID/FEI Business Operations Gerolymatos International 423075886 MANUFACTURE