Label: VAPORIZING COLDS RUB- menthol ointment
- NDC Code(s): 61734-030-02, 61734-030-03, 61734-030-04, 61734-030-05
- Packager: Delon Laboratories (1990) Ltd
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 5, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
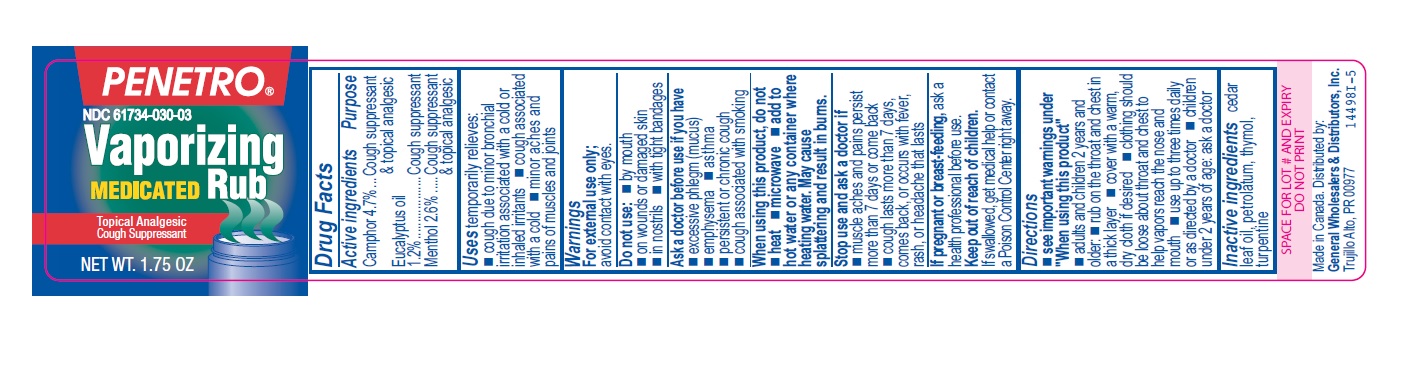
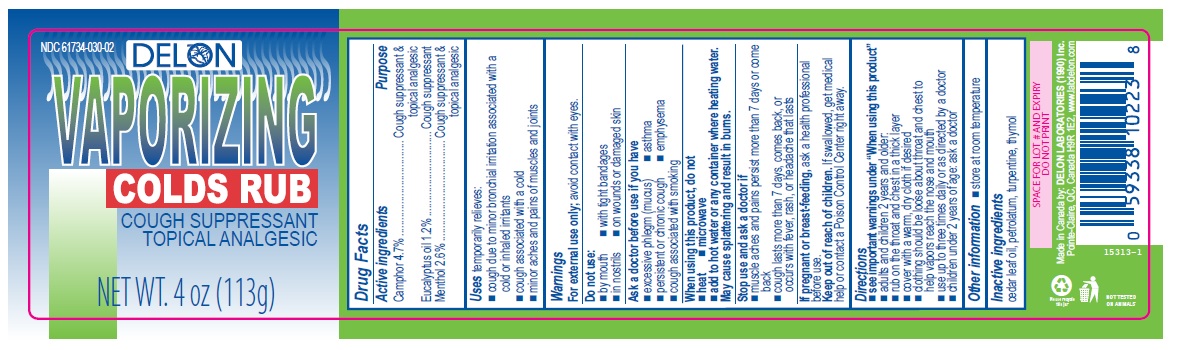
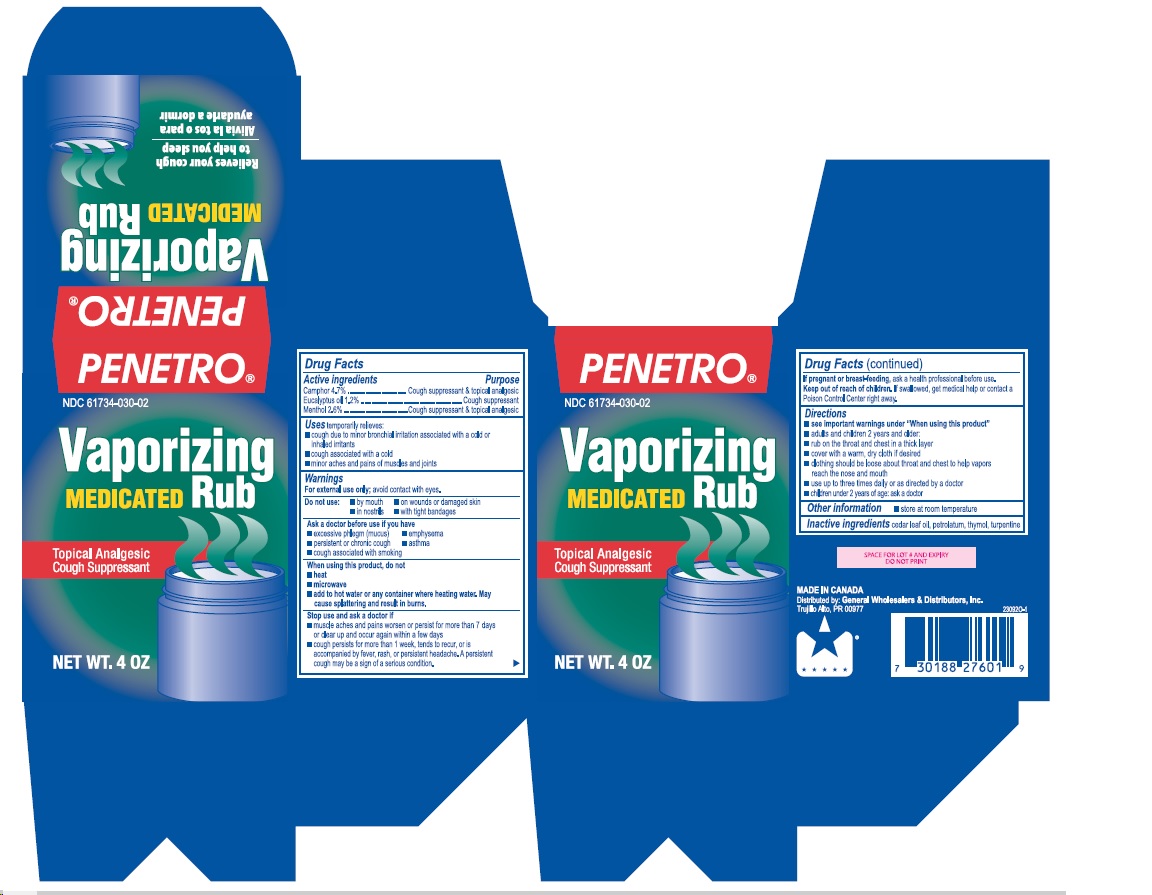
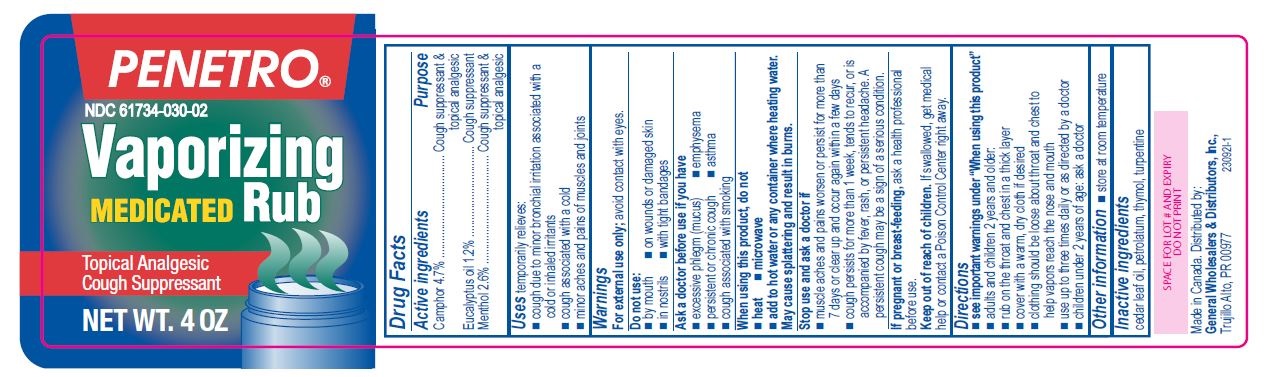
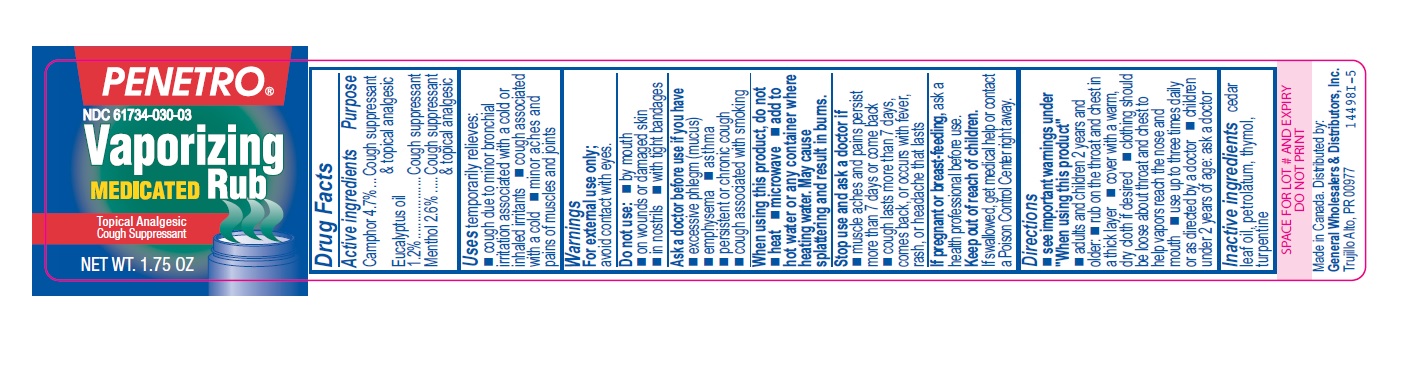
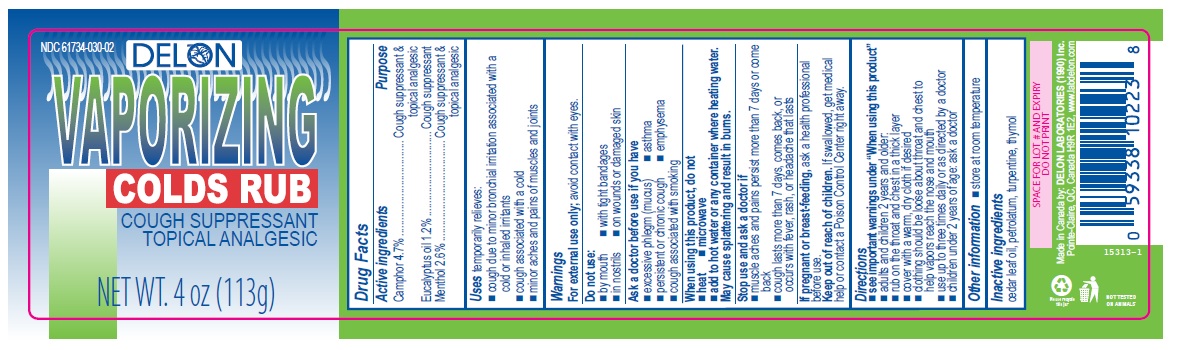
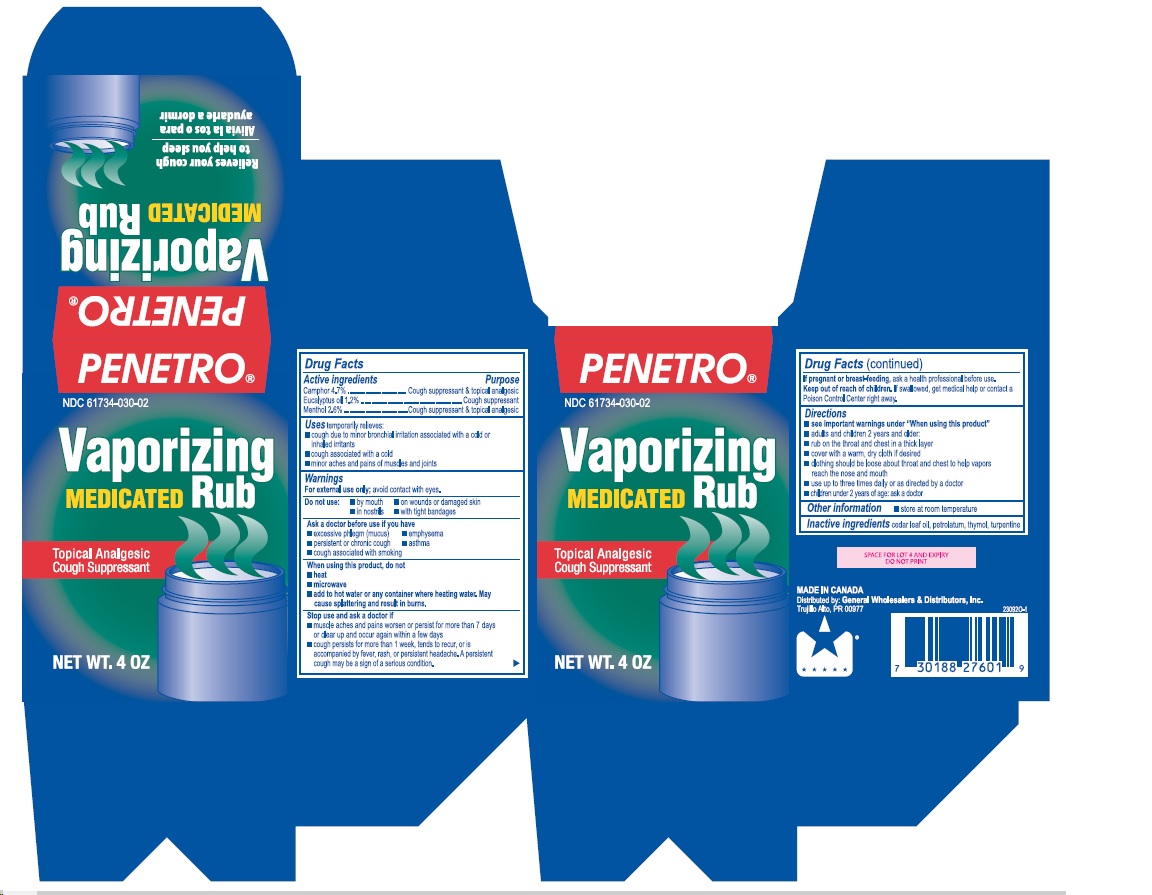
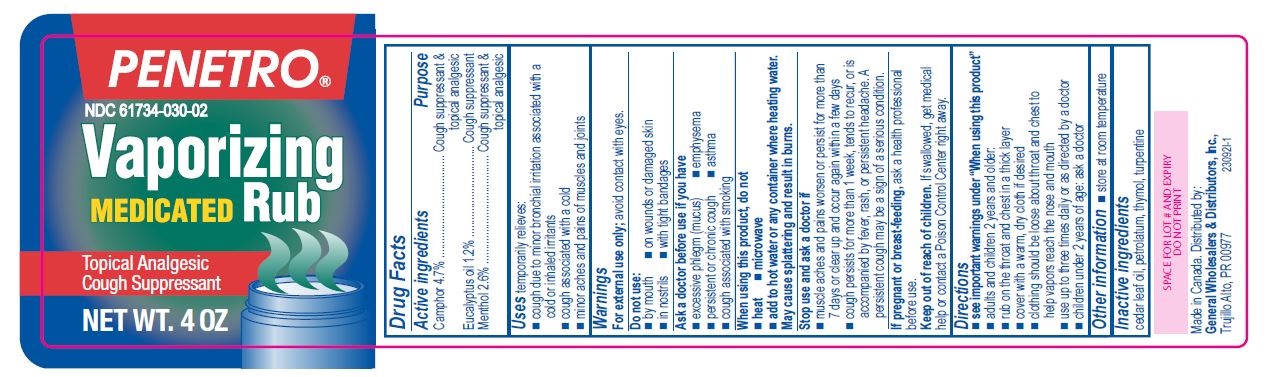
- Active ingredients
- Purpose
- Uses
-
Warnings
For external use only; avoid contact with eyes.
Ask a doctor before use if you have
- excessive phlegm (mucus)
- emphysema
- persistent or chronic cough
- asthma
- cough associated with smoking
When using this product, do not
- heat
- microwave
- add to hot water or any container where heating water. May cause splattering and result in burns.
Stop use and ask a doctor if
- muscle aches and pains worsen or persist for more than 7 days or clear up and occur again within a few days
- cough perists for more than 1 week, tends to recur, or is accompanied by fever, rash, or persistent headache. A persistent cough may be a sign of a serious condition.
-
Directions
- see important warnings under "When using this product"
- adults and children 2 years and older:
- rub on the throat and chest in a thick layer
- cover with a warm, dry cloth if desired
- clothing should be loose about throat and chest to help vapors reach the nose and mouth
- use up to three times daily or as directed by a doctor
- children under 2 years of age: ask a doctor
- Other information
- Inactive ingredients
- Penetro 1.75oz
- Delon 4oz (113g)
- Penetro 4oz
-
INGREDIENTS AND APPEARANCE
VAPORIZING COLDS RUB
menthol ointmentProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:61734-030 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 2.6 g in 100 g EUCALYPTUS OIL (UNII: 2R04ONI662) (EUCALYPTUS OIL - UNII:2R04ONI662) EUCALYPTUS OIL 1.2 g in 100 g CAMPHOR (SYNTHETIC) (UNII: 5TJD82A1ET) (CAMPHOR (SYNTHETIC) - UNII:5TJD82A1ET) CAMPHOR (SYNTHETIC) 4.73 g in 100 g Inactive Ingredients Ingredient Name Strength PETROLATUM (UNII: 4T6H12BN9U) CEDAR LEAF OIL (UNII: BJ169U4NLG) THYMOL (UNII: 3J50XA376E) TURPENTINE (UNII: XJ6RUH0O4G) Product Characteristics Color blue Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:61734-030-04 90 g in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 05/24/2011 12/04/2014 2 NDC:61734-030-02 113 g in 1 JAR; Type 0: Not a Combination Product 05/24/2011 3 NDC:61734-030-03 50 g in 1 JAR; Type 0: Not a Combination Product 05/24/2011 4 NDC:61734-030-05 85 g in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 06/22/2018 10/20/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 05/24/2011 Labeler - Delon Laboratories (1990) Ltd (248364184) Establishment Name Address ID/FEI Business Operations Laboratoires Delon 208896216 label(61734-030) , manufacture(61734-030) , pack(61734-030)