Label: PREGNYL- choriogonadotropin alfa kit
- NDC Code(s): 0052-0315-10
- Packager: Merck Sharp & Dohme LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated March 2, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
Chorionic gonadotropin, a gonadotropin, is a polypeptide hormone produced by the human placenta and obtained from the urine of pregnant persons. Chorionic gonadotropin is a purified preparation composed of an alpha and a beta subunit. The alpha subunit is essentially identical to the alpha subunits of the human pituitary gonadotropins, luteinizing hormone (LH) and follicle-stimulating hormone (FSH), as well as to the alpha subunit of human thyroid-stimulating hormone (TSH). The beta subunits of these hormones differ in amino acid sequence.
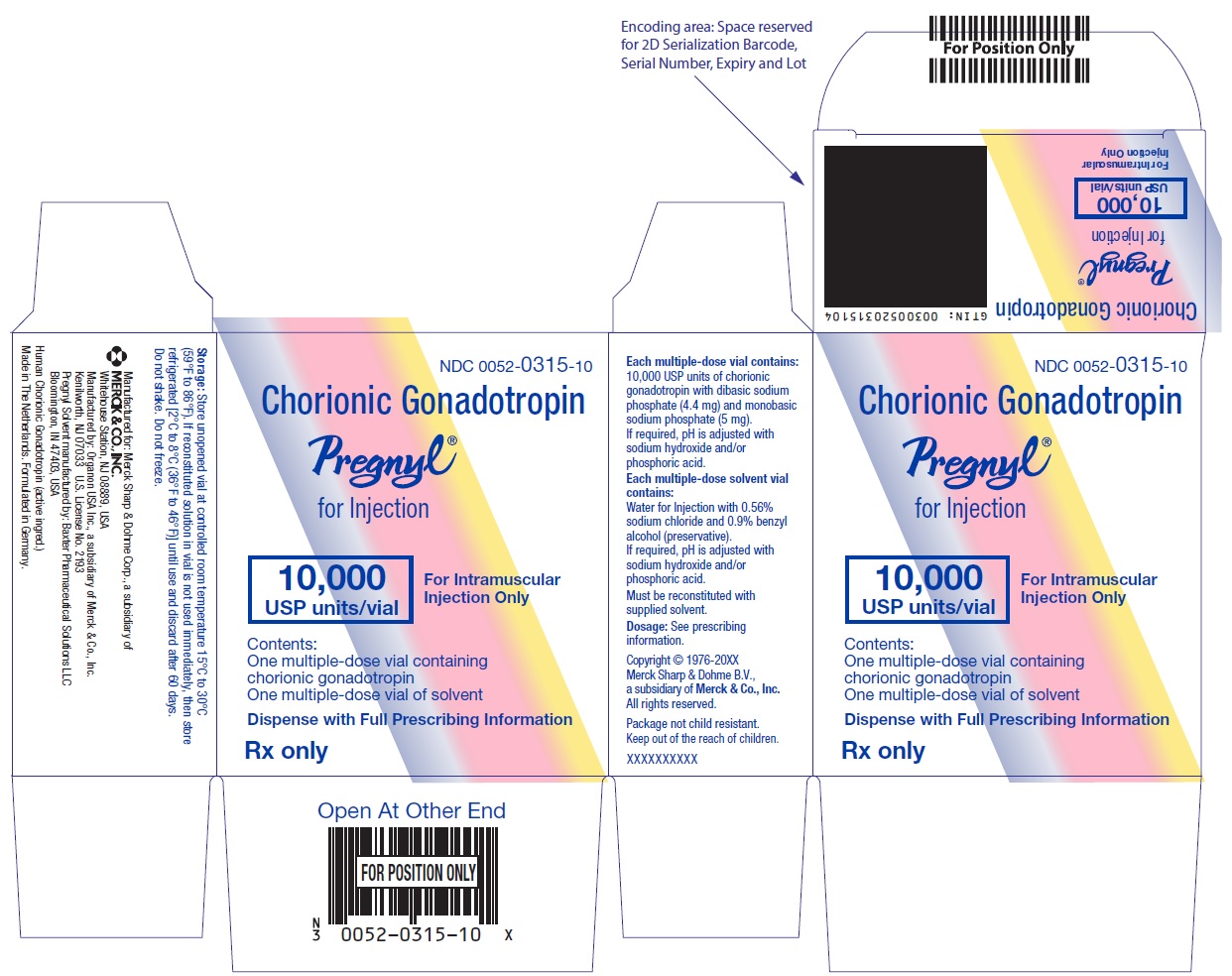
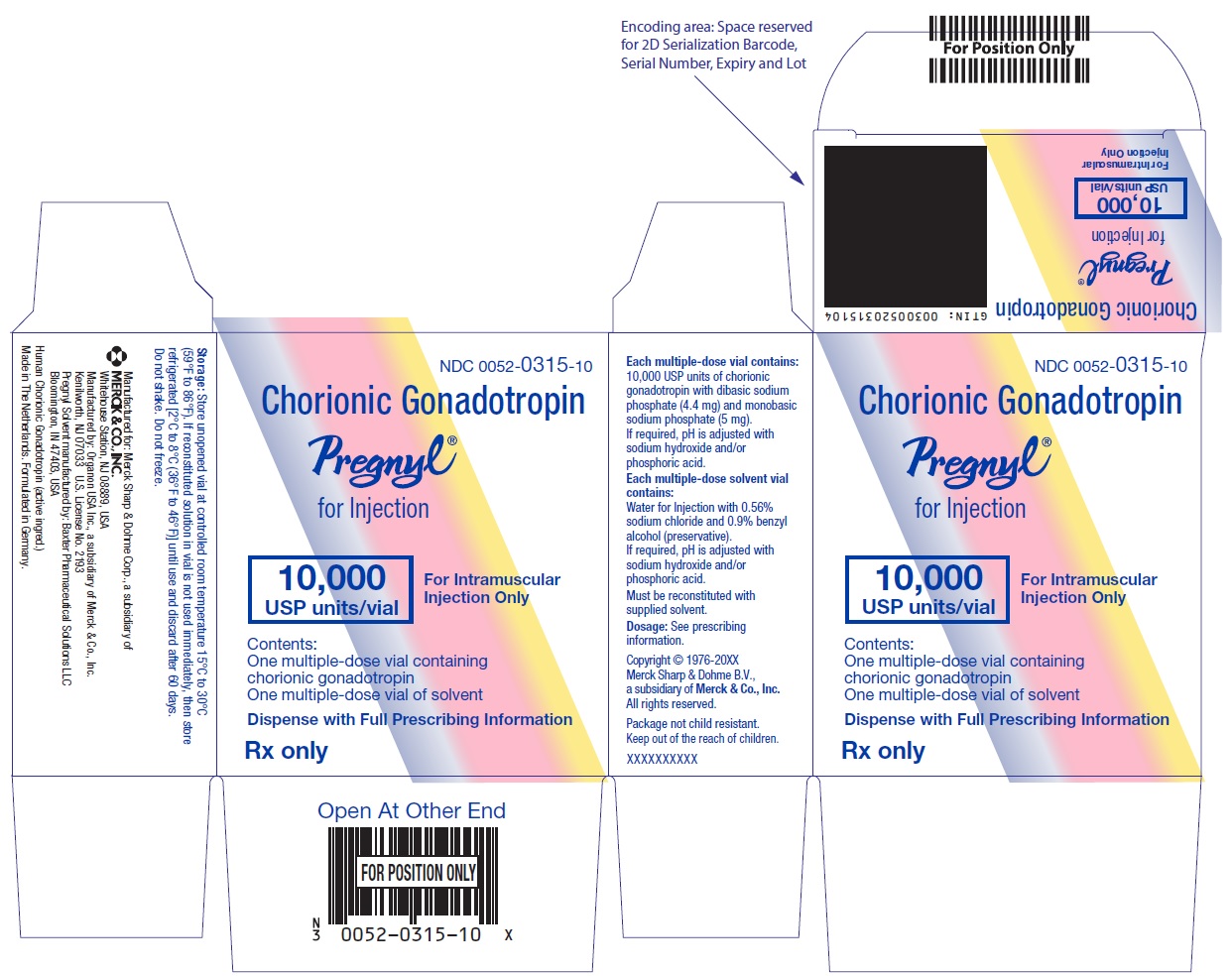
PREGNYL® (chorionic gonadotropin) for injection is a sterile, dried powder for intramuscular injection after reconstitution. Each multiple-dose vial is only for use in one patient and contains 10,000 USP units of chorionic gonadotropin with dibasic sodium phosphate (4.4 mg) and monobasic sodium phosphate (5 mg). If required, pH is adjusted with sodium hydroxide and/or phosphoric acid.
Each package also contains a 10-mL vial of solvent containing: water for injection with 0.56% sodium chloride and 0.9% BENZYL ALCOHOL (preservative), WHICH IS NOT FOR USE IN NEWBORNS. If required, pH is adjusted with sodium hydroxide and/or hydrochloric acid.
-
CLINICAL PHARMACOLOGY
The action of human chorionic gonadotropin (hCG) is virtually identical to that of pituitary LH, although hCG appears to have a small degree of FSH activity as well. It stimulates production of gonadal steroid hormones by stimulating the interstitial cells (Leydig cells) of the testis to produce androgens and the corpus luteum of the ovary to produce progesterone.
Androgen stimulation in the male leads to the development of secondary sex characteristics and may stimulate testicular descent when no anatomical impediment to descent is present. This descent is usually reversible when hCG is discontinued.
During the normal menstrual cycle, LH participates with FSH in the development and maturation of the normal ovarian follicle, and the mid-cycle LH surge triggers ovulation. hCG can substitute for LH in this function. During a normal pregnancy, hCG secreted by the placenta maintains the corpus luteum after LH secretion decreases, supporting continued secretion of estrogen and progesterone and preventing menstruation.
HCG HAS NO KNOWN EFFECT ON FAT MOBILIZATION, APPETITE OR SENSE OF HUNGER, OR BODY FAT DISTRIBUTION.
-
INDICATIONS AND USAGE
HCG HAS NOT BEEN DEMONSTRATED TO BE EFFECTIVE ADJUNCTIVE THERAPY IN THE TREATMENT OF OBESITY. THERE IS NO SUBSTANTIAL EVIDENCE THAT IT INCREASES WEIGHT LOSS BEYOND THAT RESULTING FROM CALORIC RESTRICTION, THAT IT CAUSES A MORE ATTRACTIVE OR “NORMAL” DISTRIBUTION OF FAT, OR THAT IT DECREASES THE HUNGER AND DISCOMFORT ASSOCIATED WITH CALORIE-RESTRICTED DIETS.
- Prepubertal cryptorchidism not due to anatomical obstruction. In general, hCG is thought to induce testicular descent in situations when descent would have occurred at puberty. hCG thus may help predict whether or not orchiopexy will be needed in the future. Although, in some cases, descent following hCG administration is permanent, in most cases, the response is temporary. Therapy is usually instituted in children between the ages of 4 and 9.
- Selected cases of hypogonadotropic hypogonadism (hypogonadism secondary to a pituitary deficiency) in males.
- Induction of ovulation and pregnancy in the anovulatory, infertile woman in whom the cause of anovulation is secondary and not due to primary ovarian failure, and who has been appropriately treated with human gonadotropins.
-
CONTRAINDICATIONS
- Prior hypersensitivity reactions to human gonadotropins, including hCG, or any of the excipients (see ADVERSE REACTIONS).
- High serum FSH, indicating primary gonadal failure in women.
- Presence of uncontrolled non-gonadal endocrinopathies (e.g., thyroid, adrenal, or pituitary disorders).
- Tumors of the hypothalamus or pituitary gland and ovary, breast, or uterus in females and breast or prostate in males.
- Malformations of the reproductive organs incompatible with pregnancy.
- Fibroid tumors of the uterus incompatible with pregnancy.
- Abnormal vaginal bleeding of undetermined origin.
-
WARNINGS
Use hCG in conjunction with gonadotropin therapy only if the physician is experienced with infertility problems and is familiar with the criteria for patient selection, contraindications, warnings, precautions, and adverse reactions described in the package insert for gonadotropins. Gonadotropin therapy, including hCG, requires a certain time commitment by physicians and supportive health professionals, and requires the availability of appropriate monitoring facilities (see PRECAUTIONS/Laboratory Tests). Safe and effective induction of ovulation with use of PREGNYL requires monitoring of ovarian response with serum estradiol and transvaginal ultrasound on a regular basis.
-
Anaphylaxis
Anaphylaxis has been reported with urinary-derived hCG products.
-
Ovarian Hyperstimulation Syndrome (OHSS)
Ovarian Hyperstimulation Syndrome (OHSS) is a medical event distinct from uncomplicated ovarian enlargement and may progress rapidly to become a serious medical event. OHSS is characterized by a dramatic increase in vascular permeability, which can result in a rapid accumulation of fluid in the peritoneal cavity, thorax, and potentially, the pericardium. The early warning signs of the development of OHSS are severe pelvic pain, nausea, vomiting, and weight gain. Abdominal pain, abdominal distension, gastrointestinal symptoms including nausea, vomiting and diarrhea, severe ovarian enlargement, weight gain, dyspnea, and oliguria have been reported with OHSS. Clinical evaluation may reveal hypovolemia, hemoconcentration, electrolyte imbalances, ascites, hemoperitoneum, pleural effusions, hydrothorax, acute pulmonary distress, and thromboembolic reactions. Transient liver function test abnormalities suggestive of hepatic dysfunction with or without morphologic changes on liver biopsy, have been reported in association with OHSS. OHSS occurs after gonadotropin treatment has been discontinued and it can develop rapidly, reaching its maximum about seven to ten days following treatment. Usually, OHSS resolves spontaneously with the onset of menses.
If there is evidence that OHSS may be developing prior to hCG administration, withhold hCG. Cases of OHSS are more common, more severe, and more protracted if pregnancy occurs; therefore, assess women for the development of OHSS for at least two weeks after hCG administration.
Severe OHSS may be life-threatening.
Monitor women undergoing ovarian stimulation for early signs and symptoms of OHSS. Women with known risk factors for a high ovarian response may be especially prone to the development of OHSS during or following treatment with PREGNYL.
Adherence to the recommended PREGNYL dose and treatment regimen and careful monitoring of ovarian response is important to reduce the risk of OHSS.
If serious OHSS occurs, stop gonadotropins, including hCG, and consider whether the woman should be hospitalized. Treatment is primarily symptomatic and overall consists of bed rest, fluid and electrolyte management, and analgesics (if needed). Because the use of diuretics can accentuate the diminished intravascular volume, avoid diuretics except in the late phase of resolution.
Initiate early consultation with a physician experienced in the management of OHSS and fluid and electrolyte imbalances.
-
Pulmonary and Vascular Complications
Serious pulmonary conditions (e.g., atelectasis, acute respiratory distress syndrome) have been reported in women treated with gonadotropins. In addition, thromboembolic reactions both in association with, and separate from OHSS have been reported following gonadotropin therapy. Intravascular thrombosis, which may originate in venous or arterial vessels, can result in reduced blood flow to vital organs or the extremities. Women with generally recognized risk factors for thrombosis, such as a personal or family history, severe obesity, or thrombophilia, may have an increased risk of venous or arterial thromboembolic events, during or following treatment with gonadotropins. Sequelae of such reactions have included venous thrombophlebitis, pulmonary embolism, pulmonary infarction, cerebral vascular occlusion (stroke), and arterial occlusion resulting in loss of limb and rarely in myocardial infarction. In rare cases, pulmonary complications and/or thromboembolic reactions have resulted in death. In women with recognized risk factors, the benefits of ovulation induction, in vitro fertilization (IVF) or intracytoplasmic sperm injection (ICSI) treatment need to be weighed against the risks. Pregnancy itself also carries an increased risk of thrombosis.
-
Ovarian Torsion
Ovarian torsion has been reported after treatment with gonadotropins, including PREGNYL. Ovarian torsion may be related to other conditions, such as OHSS, pregnancy, previous abdominal surgery, past history of ovarian torsion, and previous or current ovarian cysts. Damage to the ovary due to reduced blood supply can be limited by early diagnosis and immediate detorsion.
-
Multi-Fetal Gestation and Birth
Multi-fetal gestation and births have been reported with all gonadotropin therapy including hCG. Advise women of the potential risk of multiple births before starting treatment with gonadotropins including PREGNYL.
-
Congenital Malformations
The incidence of congenital malformations after ART may be slightly higher than after spontaneous conceptions. This slightly higher incidence is thought to be related to differences in parental characteristics (e.g., maternal age, sperm characteristics) and to the higher incidence of multiple gestations after ART. There are no indications that the use of gonadotropins during ART is associated with an increased risk of congenital malformations.
-
Ectopic Pregnancy
Infertile women undergoing Assisted Reproductive Technologies (ART) have an increased incidence of ectopic pregnancy. Early ultrasound confirmation that a pregnancy is intrauterine is therefore important.
-
Spontaneous Abortion
The risk of spontaneous abortion (miscarriage) is increased with gonadotropin products. However, causality has not been established. The increased risk may be a factor of the underlying infertility.
-
Ovarian Neoplasms
There have been infrequent reports of ovarian neoplasms, both benign and malignant, in women who have undergone multiple drug therapy for ovarian stimulation; however, a causal relationship has not been established.
-
Anaphylaxis
-
PRECAUTIONS
-
General
Induction of androgen secretion by hCG may cause fluid retention.
Use hCG with caution in patients with cardiac or renal disease, hypertension, epilepsy, migraine, or asthma.
Careful attention should be given to the diagnosis of infertility in candidates for hCG therapy (see INDICATIONS AND USAGE).
Evaluate patients for uncontrolled non-gonadal endocrinopathies (e.g., thyroid, adrenal or pituitary disorders) and provide the appropriate specific treatment.
-
Information for Patients
Prior to therapy with hCG, patients should be informed of the duration of treatment and monitoring of their condition that will be required. The risks of Ovarian Hyperstimulation Syndrome and multiple births in women (see WARNINGS) and other possible adverse reactions (see ADVERSE REACTIONS) should also be discussed.
-
Laboratory Tests
In most instances, treatment of women with FSH results only in follicular recruitment and development. In the absence of an endogenous LH surge, hCG is given when monitoring of the patient indicates that sufficient follicular development has occurred. This may be estimated by pelvic ultrasound alone or in combination with measurement of serum estradiol levels. The combination of both pelvic ultrasound and serum estradiol measurement are useful for monitoring the development of follicles, for timing of the ovulatory trigger, as well as for detecting ovarian enlargement and minimizing the risk of the Ovarian Hyperstimulation Syndrome and multiple gestation. Confirm the number of growing follicles using ultrasonography because serum estrogens do not give an indication of the size or number of follicles.
Human chorionic gonadotropins can crossreact in the radioimmunoassay of gonadotropins, especially luteinizing hormone. Each individual laboratory should establish the degree of crossreactivity with their gonadotropin assay. Make the laboratory aware that the patient is on hCG, if gonadotropin levels are requested.
The clinical confirmation of ovulation, with the exception of pregnancy, is obtained by direct and indirect indices of progesterone production as well as sonographic evidence of ovulation. The indices most generally used are as follows:
- A rise in basal body temperature
- Increase in serum progesterone and
- Menstruation following a shift in basal body temperature
- Urinary or serum luteinizing hormone (LH) rise
When used in conjunction with the indices of progesterone production, sonographic visualization of the ovaries will assist in determining if ovulation has occurred. Sonographic evidence of ovulation may include the following:
- Fluid in the cul-de-sac
- Collapsed follicle
- Features consistent with corpus luteum formation (e.g., ovarian stigmata, secretory endometrium)
Accurate interpretation of the indices of ovulation requires a physician who is experienced in the interpretation of these tests.
Sonographic evaluation of the early pregnancy is also important to rule out ectopic pregnancy.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term studies in animals have not been performed to evaluate the carcinogenic or mutagenic potential of chorionic gonadotropin.
Pregnancy
PREGNYL is not indicated in pregnancy. PREGNYL may be used for luteal phase support, but is discontinued upon confirmation of pregnancy. There are no data on the use of hCG in pregnancy.
In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Pediatric Use
Induction of androgen secretion by hCG may induce precocious puberty in pediatric patients treated for cryptorchidism. Discontinue therapy if signs of precocious puberty occur.
Lactation
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised if hCG is administered to a nursing woman.
The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for PREGNYL and any potential adverse effects on the breastfed child from PREGNYL or from the underlying maternal condition.
-
General
-
ADVERSE REACTIONS
For males and females
Immune system disorders
Hypersensitivity reactions, both localized and systemic in nature, including anaphylaxis have been reported. In rare cases, generalized rash or fever may occur (see CONTRAINDICATIONS and WARNINGS). If a hypersensitivity reaction is suspected, discontinue PREGNYL and assess for other potential causes for the event.Psychiatric disorders
Irritability
Restlessness
DepressionNervous system disorders
HeadacheGeneral disorders and administration site conditions
PREGNYL may cause reactions at the site of injection, such as bruising, pain, redness, swelling and itching. Occasionally, allergic reactions have been reported, mostly manifesting as pain and/or rash at the injection site (see CONTRAINDICATIONS and WARNINGS).
Edema
FatigueIn the female:
Vascular disorders
In rare instances, thromboembolism has been associated with FSH/hCG therapy, usually associated with severe OHSS (see WARNINGS).Respiratory, thoracic and mediastinal disorders
Hydrothorax, as a complication of severe OHSS (see WARNINGS).Gastrointestinal disorders
Abdominal pain and gastrointestinal symptoms such as nausea and diarrhea, related to mild OHSS. Ascites, as a complication of severe OHSS (see WARNINGS).Reproductive system and breast disorders
Unwanted ovarian hyperstimulation, mild or severe Ovarian Hyperstimulation Syndrome (see WARNINGS).
Mild to moderate enlargement of ovaries and ovarian cysts related to mild OHSS. Large ovarian cysts (prone to rupture), usually associated with severe OHSS (see WARNINGS).
Painful breastsInvestigations
Weight gain as a characteristic of severe OHSS (see WARNINGS).In the male:
Metabolism and nutrition disorders
Water and sodium retention is occasionally seen after administration of high dosages; this is regarded as a result of excessive androgen production.Reproductive system and breast disorders
HCG treatment may sporadically cause gynecomastia.
Precocious puberty -
DOSAGE AND ADMINISTRATION
For intramuscular use only. Each multiple-dose vial is to be used for one patient. The dosage regimen employed in any particular case will depend upon the indication for the use, the age and weight of the patient, and the physician’s preference. The following regimens have been advocated by various authorities:
Prepubertal cryptorchidism not due to anatomical obstruction. Generally, institute therapy in children between the ages of 4 and 9.
- 4000 USP units 3 times weekly for 3 weeks.
- 5000 USP units every second day for 4 injections.
- 15 injections for 500 to 1000 USP units over a period of 6 weeks.
- 500 USP units 3 times weekly for 4 to 6 weeks. If this course of treatment is not successful, another series is begun 1 month later, giving 1000 USP units per injection.
Selected cases of hypogonadotropic hypogonadism in males.
- 500 to 1000 USP units 3 times a week for 3 weeks, followed by the same dose twice a week for 3 weeks.
- 4000 USP units 3 times weekly for 6 to 9 months, following which the dosage may be reduced to 2000 USP units 3 times weekly for an additional 3 months.
Induction of ovulation and pregnancy in the anovulatory, infertile woman in whom the cause of anovulation is secondary and not due to primary ovarian failure and who has been appropriately treated with gonadotropins. (See also prescribing information for gonadotropins for dosage and administration for that drug product.)
5000 to 10,000 USP units 1 day following the last dose of gonadotropins. (A dosage of 10,000 USP units is recommended in the labeling for gonadotropins.)
Directions for Reconstitution
Withdraw sterile air from vial with lyophilized powder and inject into vial with solvent. Remove 1 to 10 mL of solvent and add to vial with lyophilized powder; agitate gently until powder is completely dissolved in solution. Do not shake.
PREGNYL is a white, dry powder / cake. The solvent is a clear and colorless aqueous solution.
Visually inspect parenteral drug products for particulate matter and discoloration prior to administration, whenever solution and container permit. If there is a discoloration or particulates, do not use.
IMPORTANT: IF NEEDED, THE RECONSTITUTED SOLUTION IS STABLE FOR 60 DAYS WHEN REFRIGERATED. DO NOT FREEZE. USE THE RECONSTITUTED SOLUTION WITHIN 60 DAYS OF RECONSTITUTION. IF THE RECONSTITUTED SOLUTION IS NO LONGER NEEDED, DISCARD UNUSED PORTION.
-
HOW SUPPLIED
Two-vial package containing:
One 10 mL multiple-dose vial for administration by one patient, containing lyophilized powder of 10,000 USP units of chorionic gonadotropin per vial, NDC 0052-0315-10.
One 10 mL multiple-dose vial of solvent containing: water for injection with sodium chloride 0.56% and benzyl alcohol (preservative) 0.9%, NDC 0052-0325-10. -
SPL UNCLASSIFIED SECTION
Manufactured for: Merck Sharp & Dohme Corp., a subsidiary of
MERCK & CO., INC., Whitehouse Station, NJ 08889, USA
PREGNYL manufactured by:
Organon USA Inc. a subsidiary of Merck & Co., Inc.
2000 Galloping Hill Road
Kenilworth, NJ 07033
U.S. License No. 2193
PREGNYL Solvent manufactured by:
Baxter Pharmaceutical Solutions LLC
Bloomington, IN 47403, USA
For patent information: www.msd.com/research/patent
Copyright © 1976-2023 Merck Sharp & Dohme B.V., a subsidiary of Merck & Co., Inc.
All rights reserved.uspi-mk8829-pwi-2303r008
Rx only
- PRINCIPAL DISPLAY PANEL - Kit Carton
-
INGREDIENTS AND APPEARANCE
PREGNYL
choriogonadotropin alfa kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0052-0315 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0052-0315-10 1 in 1 CARTON 10/20/1976 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 VIAL, MULTI-DOSE 10 mL Part 2 1 VIAL 10 mL Part 1 of 2 PREGNYL
choriogonadotropin alfa injection, powder, lyophilized, for solutionProduct Information Route of Administration INTRAMUSCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CHORIOGONADOTROPIN ALFA (UNII: 6413W06WR3) (CHORIOGONADOTROPIN ALFA - UNII:6413W06WR3) CHORIOGONADOTROPIN ALFA 10000 [USP'U] in 10 mL Inactive Ingredients Ingredient Name Strength SODIUM PHOSPHATE, MONOBASIC, UNSPECIFIED FORM (UNII: 3980JIH2SW) SODIUM PHOSPHATE, DIBASIC, UNSPECIFIED FORM (UNII: GR686LBA74) SODIUM HYDROXIDE (UNII: 55X04QC32I) PHOSPHORIC ACID (UNII: E4GA8884NN) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 10 mL in 1 VIAL, MULTI-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA017692 10/20/1976 Part 2 of 2 PREGNYL SOLVENT
water, sodium chloride and benzyl alcohol injection, solutionProduct Information Route of Administration INTRAMUSCULAR Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) SODIUM CHLORIDE (UNII: 451W47IQ8X) BENZYL ALCOHOL (UNII: LKG8494WBH) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 10 mL in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA017692 10/20/1976 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA017692 10/20/1976 Labeler - Merck Sharp & Dohme LLC (118446553)