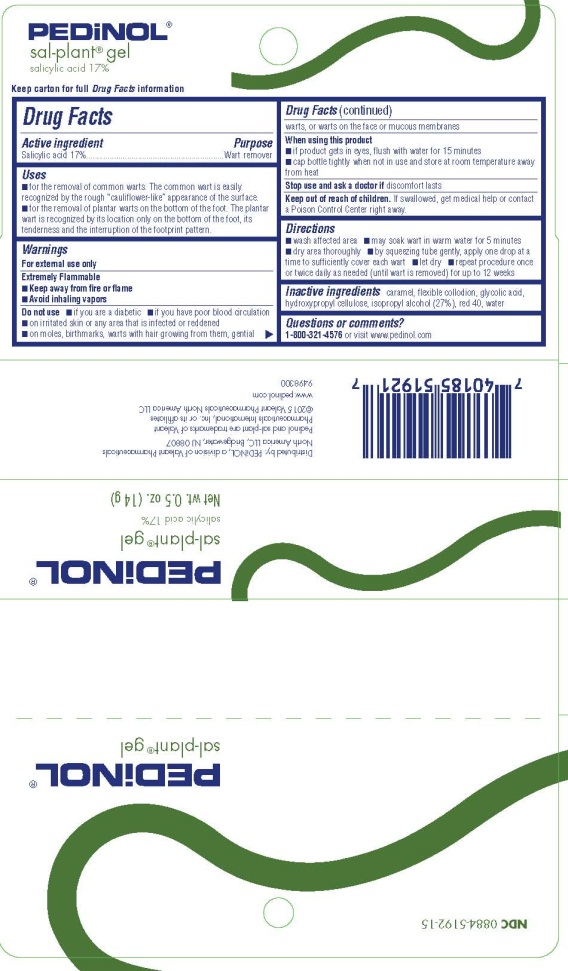
SAL-PLANT GEL- salicylic acid gel
Pedinol Pharmacal, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Uses
- •
- for the removal of common warts. The common wart is easily recognized by the rough “cauliflower-like” appearance of the surface.
- •
- for the removal of plantar warts on the bottom of the foot. The plantar wart is recognized by its location only on the bottom of the foot, its tenderness and the interruption of the footprint pattern.
Warnings
For external use only
Extremely flammable
- •
- Keep away from fire or flame
- •
- Avoid inhaling vapors
Do not use
- •
- if you are a diabetic
- •
- if you have poor blood circulation
- •
- on irritated skin or any area that is infected or reddened
- •
- on moles, birthmarks, warts with hair growing from them, genital warts, or warts on the face or mucous membranes
Directions
- •
- wash affected area
- •
- may soak wart in warm water for 5 minutes
- •
- dry area thoroughly
- •
- by squeezing tube gently, apply one drop at a time to sufficiently cover each wart
- •
- let dry
- •
- repeat procedure once or twice daily as needed (until wart is removed) for up to 12 weeks
| SAL-PLANT GEL
salicylic acid gel |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Pedinol Pharmacal, Inc. (064737125) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Cospro Development Corp | 785638821 | MANUFACTURE(0884-5192) | |
Revised: 2/2016
Document Id: 90dc0a72-af00-4810-a7c3-8a32366f155c
Set id: 3c8552fd-5f5d-4180-9d6f-752d94951925
Version: 4
Effective Time: 20160216
Pedinol Pharmacal, Inc.