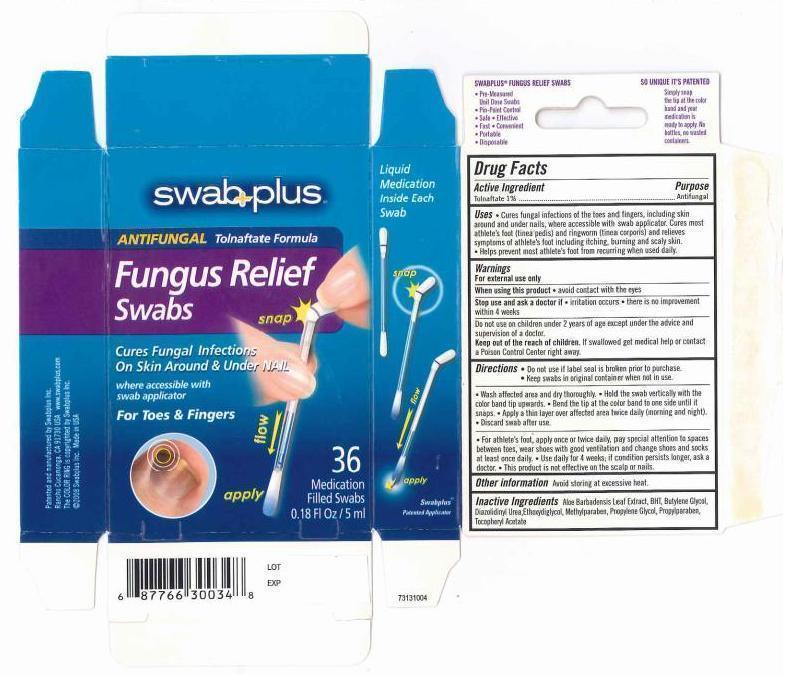
Label: FUNGUS RELIEF- tolnaftate solution
-
Contains inactivated NDC Code(s)
NDC Code(s): 65734-348-00, 65734-348-36 - Packager: Swabplus Inc.
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated January 28, 2015
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Purpose
-
Uses
- Cures fungal infections of the toes and fingers, including skin around and under nails, where accessible with swab applicator. Cures most athlete's foot (tinea pedis) and ringworm (tinea corporis) and relieves symptoms of athlete's foot including itching, burning and scaly skin.
- Help prevent most athlete's foot from recuring when uses daily.
- Warings
- Keep out of the reach of children
-
Directions
- Do not use if label seal is broken prior to purchase.
- Keep swabs in original container when not in use.
- wash affected area and dry thoroughly.
- Hold the swab vertically with the color band tip upwards.
- Bend the tip at the color band to one side until it snaps.
- Apply a thin layer over affected area twice daily (morning and night)
- Discard swab after use.
- For athlete's foot, apply once or twice daily, pay special attention to spaces between toes, wear shoes with good ventilation and change shoes and socks at least once daily.
- Use daily for 4 weeks; if condition persists longer, ask a doctor.
- This product is not effective on the scalp or nails.
- Other information
- Inactive Ingredients
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
FUNGUS RELIEF
tolnaftate solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:65734-348 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TOLNAFTATE (UNII: 06KB629TKV) (TOLNAFTATE - UNII:06KB629TKV) TOLNAFTATE 10 mg in 1 mL Inactive Ingredients Ingredient Name Strength ALOE VERA LEAF (UNII: ZY81Z83H0X) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) DIAZOLIDINYL UREA (UNII: H5RIZ3MPW4) DIETHYLENE GLYCOL MONOETHYL ETHER (UNII: A1A1I8X02B) METHYLPARABEN (UNII: A2I8C7HI9T) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) PROPYLPARABEN (UNII: Z8IX2SC1OH) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:65734-348-36 36 in 1 PACKAGE 1 NDC:65734-348-00 0.15 mL in 1 APPLICATOR Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part333C 03/01/2003 Labeler - Swabplus Inc. (876441549) Registrant - Swabplus Inc. (876441549) Establishment Name Address ID/FEI Business Operations Swabplus Inc. 876441549 manufacture(65734-348) , relabel(65734-348) , repack(65734-348)