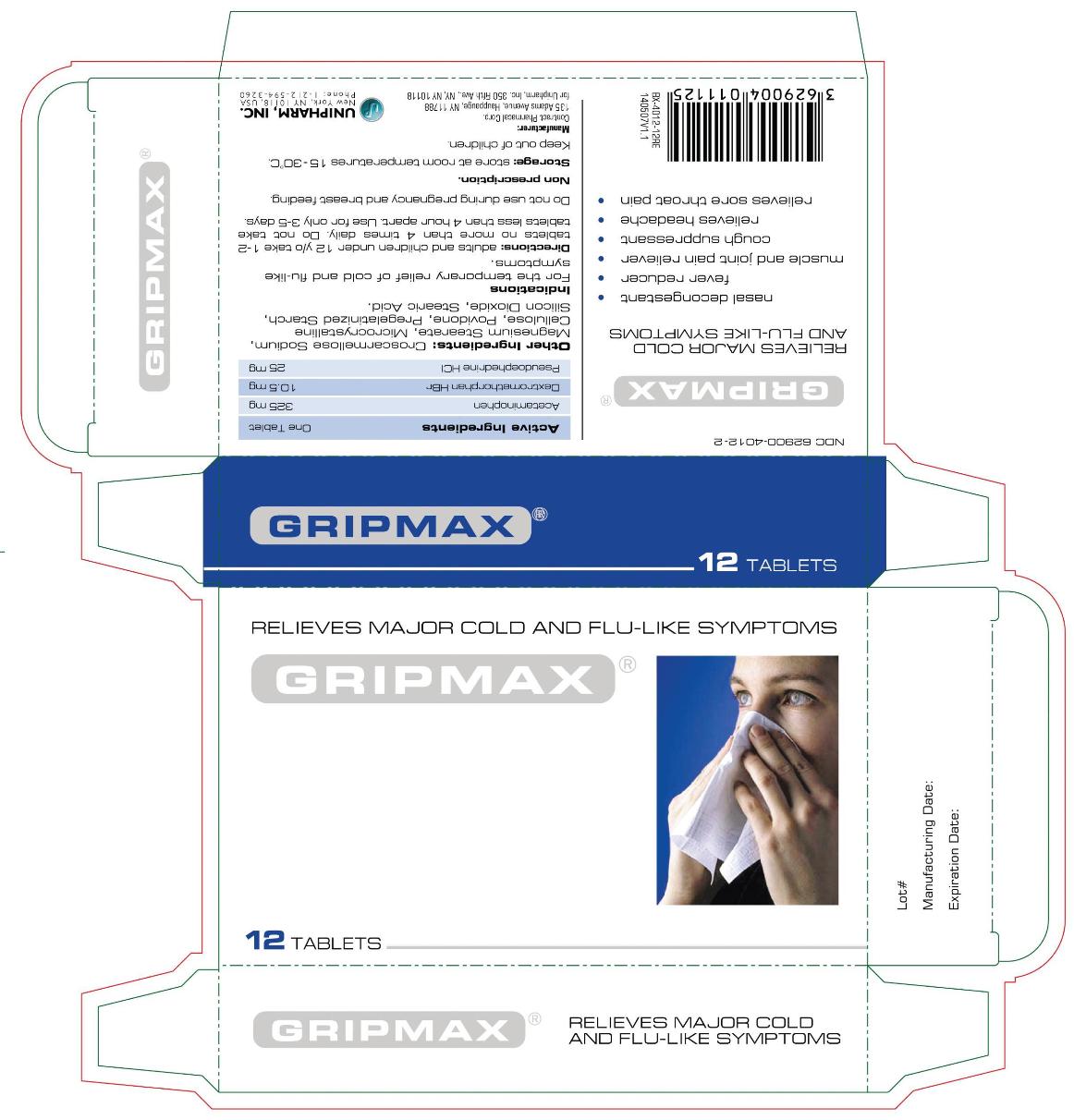
GRIPMAX- acetaminophen, pseudoephedrine hydrochloride, dextromethorphan hydrobromide tablet
Unipharm, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Gripmax Tablets
Relieves major cold and flu-like symptoms
- Nasal decongestant
- Fever reducer
- Muscle and joint pain relieve
- Cough suppressant
- Relieves headache
- Relieves sore throat pain
ACTIVE INGREDIENT
| Active Ingredients | One Tablet |
| Acetaminophen | 325 mg |
| Dextromethorphan HBr | 10.5 mg |
| Pseudoephedrine HCl | 25 mg |
Other Ingredients
Croscarmellose Sodium, Magnesium Stearate, Microcrystalline Cellulose, Povidone, Pregelatinized Starch, Silicon Dioxide, Stearic Acid.
Directions: adults and children 12 y/o take 1-2 tablets no more than 4 times daily. Do not take tablets less than 4 hours apart. Use for only 3-5 days.
| GRIPMAX
acetaminophen, pseudoephedrine hydrochloride, dextromethorphan hydrobromide tablet |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Unipharm, Inc. (884789132) |
| Registrant - Unipharm, Inc. (884789132) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Contract Pharmacal Corp | 968335112 | MANUFACTURE(62900-4012) | |
Revised: 3/2020
Document Id: a025c0e7-2924-41a4-8899-727223513dbe
Set id: 3c2c6020-445a-4b4f-a9ee-3cdaddb19ff1
Version: 3
Effective Time: 20200324
Unipharm, Inc.