Label: CHLORZOXAZONE tablet
-
Contains inactivated NDC Code(s)
NDC Code(s): 55045-1594-8 - Packager: Dispensing Solutions, Inc.
- This is a repackaged label.
- Source NDC Code(s): 0555-0585
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated September 7, 2011
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION:
Each tablet contains:
Chlorzoxazone…………500 mg
Inactive Ingredients: Colloidal silicon dioxide, croscarmellose sodium, docusate sodium, lactose anhydrous, magnesium stearate, microcrystalline cellulose, sodium benzoate, D&C yellow no. 10 aluminum lake, FD&C blue no. 1 aluminum lake HT.
5-chloro-2-benzoxazolinone. The structural formula is as follows:
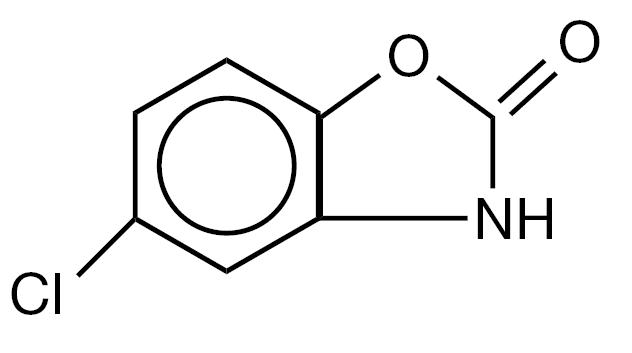
C7H4ClNO2 Molecular Weight:169.57
-
CLINICAL PHARMACOLOGY:
Chlorzoxazone is a centrally-acting agent for painful musculoskeletal conditions. Data available from animal experiments as well as human study indicate that chlorzoxazone acts primarily at the level of the spinal cord and subcortical areas of the brain where it inhibits multi-synaptic reflex arcs involved in producing and maintaining skeletal muscle spasm of varied etiology. The clinical result is a reduction of the skeletal muscle spasm with relief of pain and increased mobility of the involved muscles. Blood levels of chlorzoxazone can be detected in people during the first 30 minutes and peak levels may be reached, in the majority of the subjects, in about 1 to 2 hours after oral administration of chlorzoxazone. Chlorzoxazone is rapidly metabolized and is excreted in the urine, primarily in a conjugated form as the glucuronide. Less than one percent of a dose of chlorzoxazone is excreted unchanged in the urine in 24 hours.
-
INDICATIONS AND USAGE:
Chlorzoxazone tablets are indicated as an adjunct to rest, physical therapy, and other measures for the relief of discomfort associated with acute painful musculoskeletal conditions. The mode of action of this drug has not been clearly identified but may be related to its sedative properties. Chlorzoxazone does not directly relax tense skeletal muscles in man.
- CONTRAINDICATIONS:
-
WARNINGS:
Serious (including fatal) hepatocellular toxicity has been reported rarely in patients receiving chlorzoxazone. The mechanism is unknown but appears to be idiosyncratic and unpredictable. Factors predisposing patients to this rare event are not known. Patients should be instructed to report early signs and/or symptoms of hepatotoxicity such as fever, rash, anorexia, nausea, vomiting, fatigue, right upper quadrant pain, dark urine, or jaundice. Chlorzoxazone should be discontinued immediately and a physician consulted if any of these signs or symptoms develop. Chlorzoxazone use should also be discontinued if a patient develops abnormal liver enzymes (e.g., AST, ALT, alkaline phosphatase and bilirubin).
The concomitant use of alcohol or other central nervous system depressants may have an additive effect.
Usage in Pregnancy:
The safe use of chlorzoxazone has not been established with respect to the possible adverse effects upon fetal development. Therefore, it should be used in women of childbearing potential only when, in the judgment of the physician, the potential benefits outweigh the possible risks.
-
PRECAUTIONS:
Chlorzoxazone should be used with caution in patients with known allergies or with a history of allergic reactions to drugs. If a sensitivity reaction occurs such as urticaria, redness, or itching of the skin, the drug should be stopped.
If any symptoms suggestive of liver dysfunction are observed, the drug should be discontinued.
-
ADVERSE REACTIONS:
After extensive clinical use of chlorzoxazone-containing products, it is apparent that the drug is well-tolerated and seldom produces undesirable side effects. Occasional patients may develop gastrointestinal disturbances. It is possible in rare instances that chlorzoxazone may have been associated with gastrointestinal bleeding. Drowsiness, dizziness, lightheadedness, malaise, or overstimulation may be noted by an occasional patient. Rarely, allergic-type skin rashes, petechiae, or ecchymoses may develop during treatment. Angioneurotic edema or anaphylactic reactions are extremely rare. There is no evidence that the drug will cause renal damage. Rarely, a patient may note discoloration of the urine resulting from a phenolic metabolite of chlorzoxazone. This finding is of no known clinical significance.
-
OVERDOSAGE:
Symptoms:
Initially, gastrointestinal disturbances such as nausea, vomiting, or diarrhea together with drowsiness, dizziness, lightheadedness or headache may occur. Early in the course there may be malaise or sluggishness followed by marked loss of muscle tone, making voluntary movement impossible. The deep tendon reflexes may be decreased or absent. The sensorium remains intact, and there is no peripheral loss of sensation. Respiratory depression may occur with rapid, irregular respiration and intercostal and substernal retraction. The blood pressure is lowered, but shock has not been observed.
Treatment:
Gastric lavage or induction of emesis should be carried out, followed by administration of activated charcoal. Thereafter, treatment is entirely supportive. If respirations are depressed, oxygen and artificial respiration should be employed and a patent airway assured by use of an oropharyngeal airway or endotracheal tube. Hypotension may be counteracted by use of dextran, plasma, concentrated albumin or a vasopressor agent such as norepinephrine. Cholinergic drugs or analeptic drugs are of no value and should not be used.
- DOSAGE AND ADMINISTRATION:
-
HOW SUPPLIED:
Chlorzoxazone Tablets, USP are available as:
500 mg: Light green, round, scored tablet. Debossed with 555/585 on one side and stylized barr on the other side. Available in bottles of: 20 NDC 0555-0585-18 100 NDC 0555-0585-02 500 NDC 0555-0585-04 1000 NDC 0555-0585-05 Dispense with a child-resistant closure in a tight container as defined in the USP/NF.
Store at controlled room temperature 15º-30ºC (59º-86ºF) [See USP].
MANUFACTURED BY
BARR LABORATORIES, INC.
POMONA, NY 10970
Revised SEPTEMBER 2002
BR-585
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
CHLORZOXAZONE
chlorzoxazone tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:55045-1594(NDC:0555-0585) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CHLORZOXAZONE (UNII: H0DE420U8G) (CHLORZOXAZONE - UNII:H0DE420U8G) CHLORZOXAZONE 500 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) DOCUSATE SODIUM (UNII: F05Q2T2JA0) MAGNESIUM STEARATE (UNII: 70097M6I30) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) SODIUM BENZOATE (UNII: OJ245FE5EU) D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) ALUMINUM OXIDE (UNII: LMI26O6933) Product Characteristics Color green (light green) Score 2 pieces Shape ROUND Size 11mm Flavor Imprint Code 555;585;barr Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:55045-1594-8 30 in 1 BOTTLE, PLASTIC Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA089895 01/16/2011 Labeler - Dispensing Solutions, Inc. (066070785) Establishment Name Address ID/FEI Business Operations Dispensing Solutions, Inc. 066070785 relabel, repack


