STOMACH RELIEF CHILDRENS- calcium carbonate tablet, chewable
DOLGENCORP, LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Rexall 44-596 Delisted
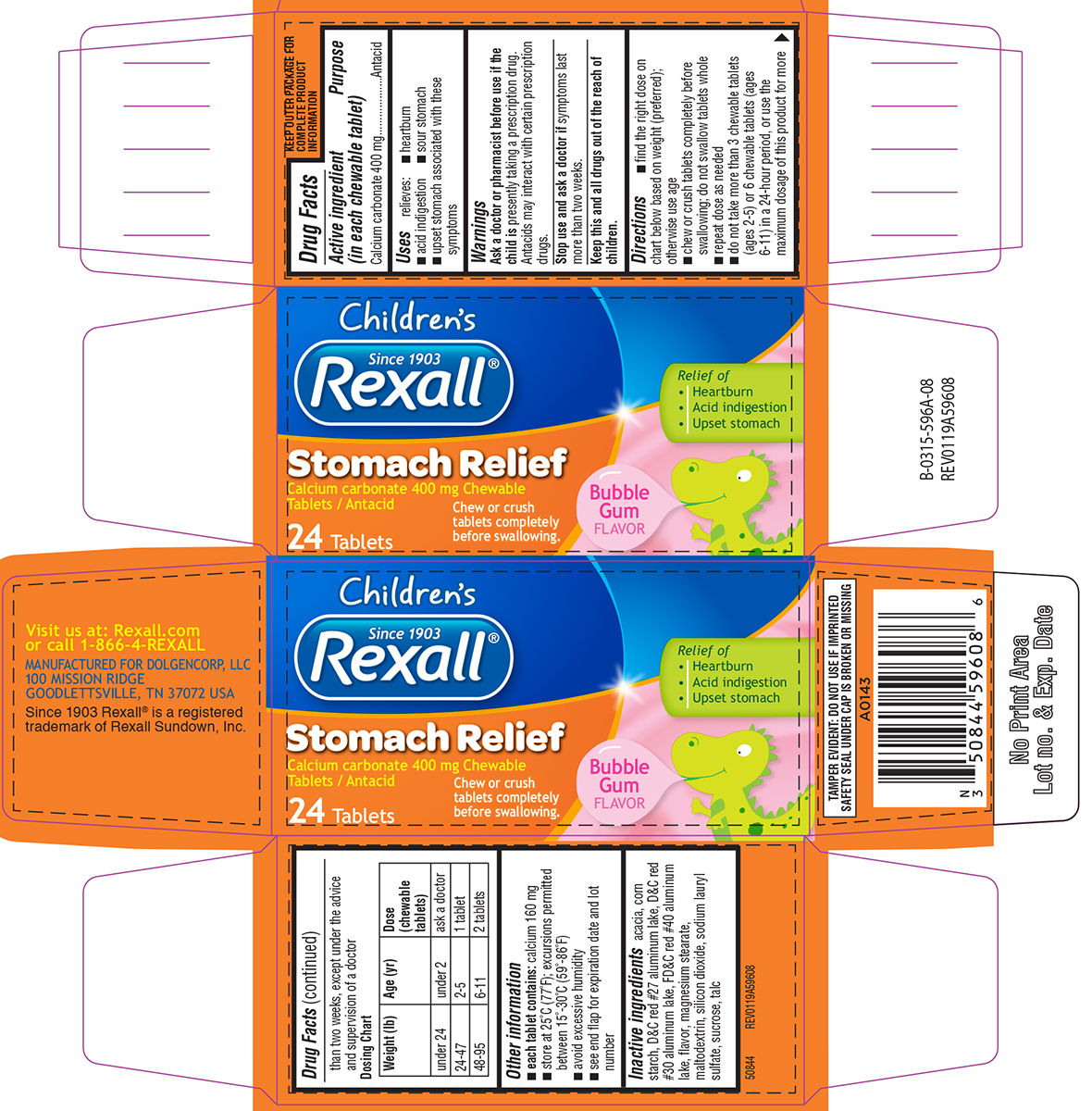
Warnings
Directions
- find the right dose on chart below based on weight (preferred); otherwise use age
- chew or crush tablets completely before swallowing; do not swallow tablets whole
- repeat dose as needed
- do not take more than 3 chewable tablets (ages 2-5) or 6 chewable tablets (ages 6-11) in a 24-hour period, or use the maximum dosage of this product for more than two weeks, except under the advice and supervision of a doctor
Dosing Chart
| Weight (lb) | Age (yr) | Dose (chewable tablets) |
| under 24 | under 2 | ask a doctor |
| 24-47 | 2-5 | 1 tablet |
| 48-95 | 6-11 | 2 tablets |
Other information
- each tablet contains: calcium 160 mg
- store at 25ºC (77ºF); excursions permitted between 15º-30ºC (59º-86ºF)
- avoid excessive humidity
- see end flap for expiration date and lot number
Inactive ingredients
acacia, corn starch, D&C red #27 aluminum lake, D&C red #30 aluminum lake, FD&C red #40 aluminum lake, flavor, magnesium stearate, maltodextrin, silicon dioxide, sodium lauryl sulfate, sucrose, talc
Principal Display Panel
Children's
Since 1903
Rexall®
Stomach Relief
Calcium carbonate 400 mg chewable Tablets / Antacid
24 Tablets
Relief of
• Heartburn
• Acid indigestion
• Upset stomach
Bubble Gum Flavor
Chew or crush tablets completely before swallowing.
TAMPER EVIDENT: DO NOT USE IF IMPRINTED
SAFETY SEAL UNDER CAP IS BROKEN OR MISSING
Visit us at: Rexall.com
or call 1-866-4-REXALL
MANUFACTURED FOR DOLGENCORP, LLC
100 MISSION RIDGE
GOODLETTSVILLE, TN 37072 USA
Since 1903 Rexall® is a registered
trademark of Rexall Sundown, Inc.
50844 REV0119A59608
Rexall 44-596A
| STOMACH RELIEF
CHILDRENS
calcium carbonate tablet, chewable |
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
| Labeler - DOLGENCORP, LLC (068331990) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 038154464 | pack(55910-596) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 832867837 | manufacture(55910-596) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 967626305 | pack(55910-596) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 117025878 | manufacture(55910-596) | |