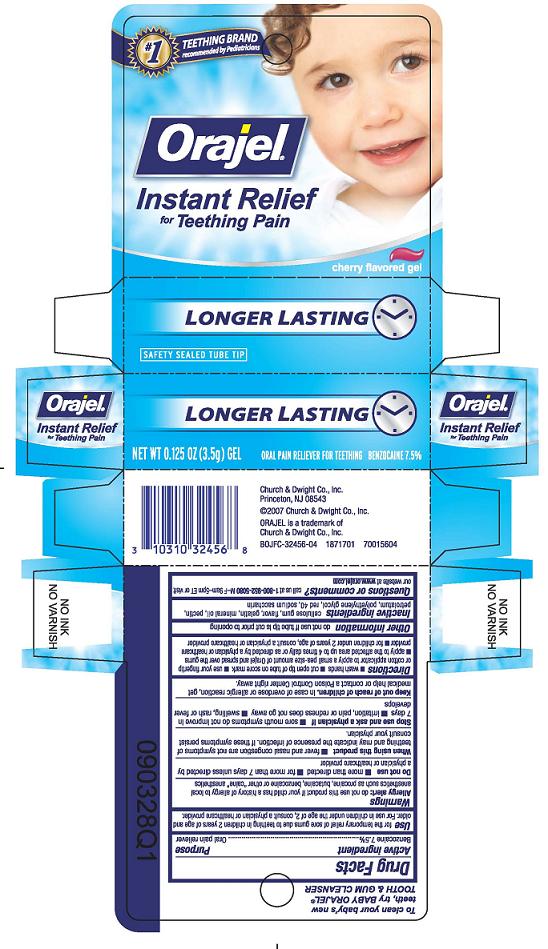
ORAJEL INSTANT RELIEF FOR TEETHING PAIN LONGER LASTING- benzocaine gel
Church & Dwight Co., Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
DRUG FACTS
Use
For the temporary relief of sore gums due to teething in children 2 years of age and older.
For use in children under the age of 2 consult a physician or healthcare provider.
Warnings
Allergy Alert:
do not use this product if your child has a history of allergy to local anesthetics such as procaine, butacaine, benzocaine or other "caine" anesthetics
Do not use
- more than directed
- for more than 7 days unless directed by a physician or healthcare provider
When using this product
fever and nasal congestion are not symptoms of teething and may indicate the presence of infection. If these symptoms persist, consult your physician.
Stop use and ask a doctor if
sore mouth symptoms do not improve in 7 days,
irritation, pain or redness does not go away,
swelling, rash or fever develops
Keep out of reach of children. In case of overdose or allergic reaction, get medical help or contact a Poison Control Center right away.
Directions
- wash hands
- cut open tip of tube on score mark
- use your fingertip or cotton applicator to apply a small pea-size amount of Orajel and spread over the gums
- apply to the affected area up to 4 times daily or as directed by a physician or healthcare provider
- for children under 2 years of age, consult a physician or healthcare provider
Inactive ingredients
cellulose gum, flavor, gelatin, mineral oil, pectin, petrolatum, polyethylene glycol, red 40, sodium saccharin
| ORAJEL INSTANT RELIEF FOR TEETHING PAIN
LONGER LASTING
benzocaine gel |
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Church & Dwight Co., Inc. (001211952) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Church & Dwight Co., Inc. | 043690812 | manufacture(10237-735) | |