Label: HEMORRODIL UNGUENTO PLUS- hydrocortisone ointment
-
Contains inactivated NDC Code(s)
NDC Code(s): 61357-132-01 - Packager: ZURICH MEDICAL LABS, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Export only
Drug Label Information
Updated March 11, 2014
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active Ingredients
- Purpose
- Uses
-
Warnings
For external use only.
When using this product: avoid contact with eyes, do not exceed the recommended daily dosage unless directed by a doctor, & do not put into the rectum by using fingers or any mechanical device or applicator.
-
Instructions
Adults
When practical, cleanse the affected area by patting or blotting with an appropriate cleansing wipe. Gently dry by patting or blotting with tissue or a soft cloth before application of this product
- STORAGE AND HANDLING
- Inactive Ingredients
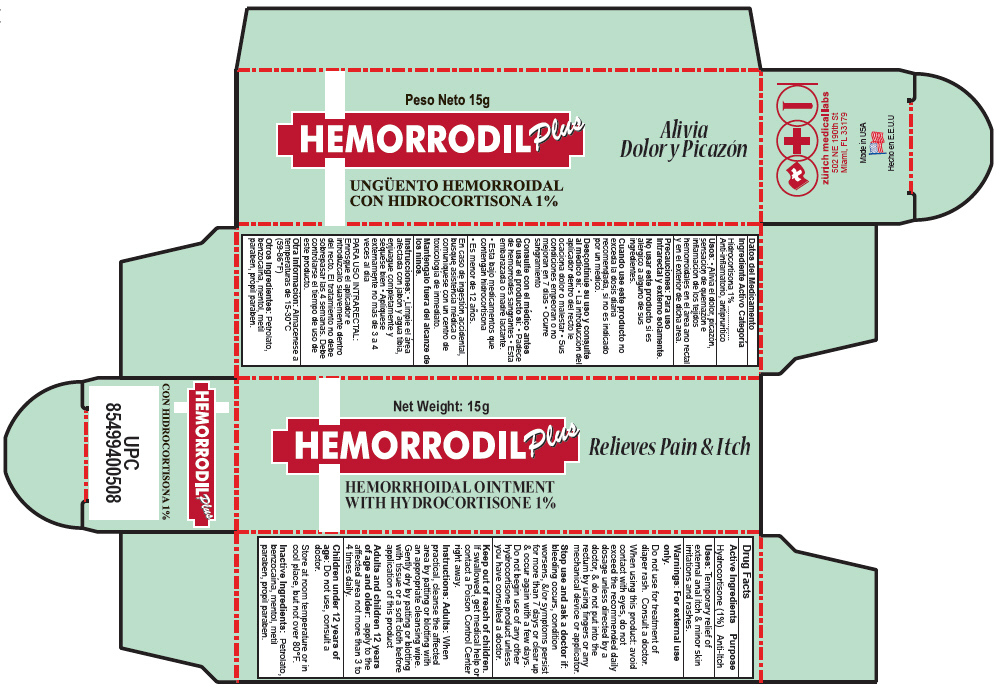
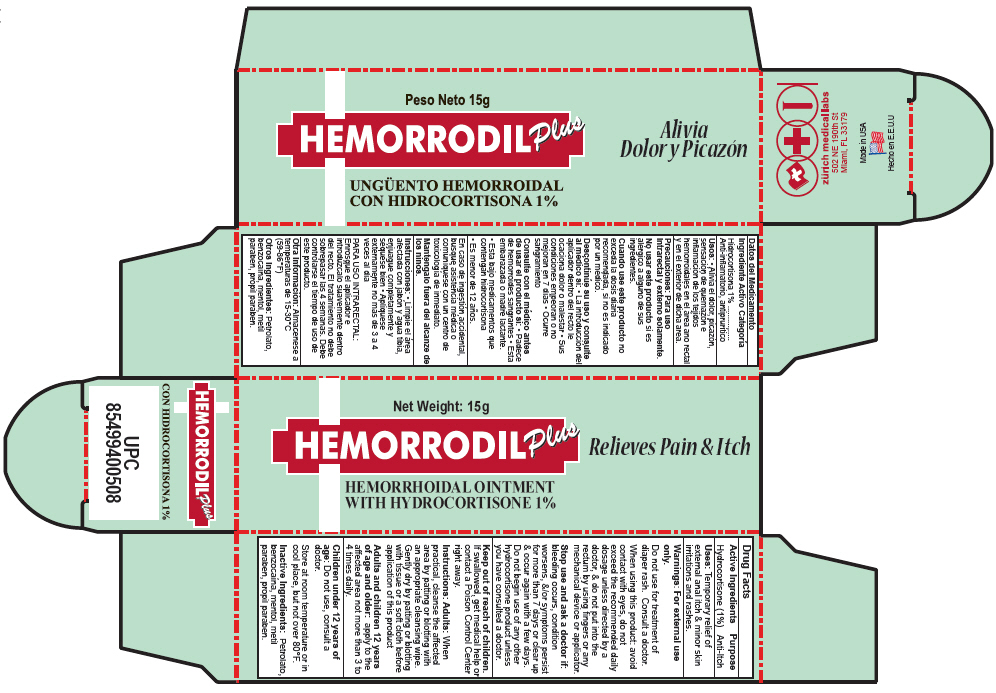
- PRINCIPAL DISPLAY PANEL - 15 g Tube Carton
-
INGREDIENTS AND APPEARANCE
HEMORRODIL UNGUENTO PLUS
hydrocortisone ointmentProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:61357-132 Route of Administration RECTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Hydrocortisone (UNII: WI4X0X7BPJ) (Hydrocortisone - UNII:WI4X0X7BPJ) Hydrocortisone 10 mg in 1 g Inactive Ingredients Ingredient Name Strength BENZOCAINE (UNII: U3RSY48JW5) PETROLATUM (UNII: 4T6H12BN9U) MENTHOL (UNII: L7T10EIP3A) METHYLPARABEN (UNII: A2I8C7HI9T) PROPYLPARABEN (UNII: Z8IX2SC1OH) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:61357-132-01 1 in 1 CARTON 1 15 g in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date EXPORT ONLY 03/01/1964 Labeler - ZURICH MEDICAL LABS, LLC (071904097) Establishment Name Address ID/FEI Business Operations ZURICH MEDICAL LABS, LLC 071904097 MANUFACTURE(61357-132)