ATROPINE SULFATE- atropine sulfate solution
Bausch & Lomb Incorporated
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
Atropine Sulfate Ophthalmic Solution USP, 1% (Sterile)
Rx only
DESCRIPTION
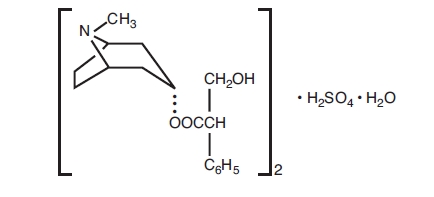
Atropine Sulfate Ophthalmic Solution USP, 1% is a sterile topical anticholinergic for ophthalmic use. The active ingredient is represented by the chemical structural formula:
(C17H23NO3)2•H2SO4•H2O
Mol. wt. 694.84
Chemical name:
Benzeneacetic acid, α-(hydroxymethyl)-, 8-methyl-8-azabicyclo-(3.2.1)oct-3-yl ester, endo-(±)-, sulfate (2:1) (salt), monohydrate.
Each mL Contains:
Atropine Sulfate Ophthalmic Solution USP, 1%, contains in each mL of solution: ACTIVE: Atropine Sulfate USP, 1% (10mg); INACTIVES: Boric Acid, Hypromellose and Purified Water; Sodium Hydroxide and/or Hydrochloric Acid may be added to adjust pH. PRESERVATIVE ADDED: Benzalkonium Chloride 0.01%.
CHEMICAL PHARMACOLOGY
The anticholinergic effect of this product blocks the responses of the sphincter muscle of the iris and the accommodative muscle of the ciliary body to cholinergic stimulation, producing pupillary dilation (mydriasis) and paralysis of accommodation (cycloplegia).
INDICATIONS AND USAGE
For mydriasis and/or cycloplegia. For cycloplegic refraction, for pupillary dilation desired in inflammatory conditions of the iris and uveal tract.
CONTRAINDICATIONS
This product should not be used in patients with primary glaucoma or a predisposition to narrow anterior chamber angle glaucoma. This product should not be used in pediatric patients who have previously had a severe systemic reaction to atropine. This product should not be used in those persons showing hypersensitivity to any component of this preparation.
WARNINGS
Not for injection into the eye. Do not touch dropper tip to any surface, as this may contaminate the solution. In pediatric patients, use with extreme caution. Excessive use in pediatric patients or in certain individuals with a previous history of susceptibility to belladonna alkaloids may produce systemic symptoms of atropine poisoning. If this occurs, discontinue medication, and use appropriate therapy as outlined in “OVERDOSAGE” section.
PRECAUTIONS
To avoid excessive systemic absorption, the lacrimal sac should be compressed by digital pressure for two to three minutes after instillation. To avoid inducing angle closure glaucoma, an estimation of the depth of the angle of the anterior chamber should be made. Administration of atropine in infants requires great caution.
Patient Warning:
Patients should be advised not to drive or engage in other hazardous activities while pupils are dilated. Patients may experience sensitivity to light and should protect eyes in bright illumination during dilation.
Parents should be warned not to get this preparation in their children’s mouth and to wash their own hands and the child’s hands following administration.
Carcinogenesis, Mutagenesis, Impairment of Fertility:
No studies have been conducted in animals or in humans to evaluate the potential of these effects.
ADVERSE REACTIONS
Prolonged use may produce local irritation characterized by follicular conjunctivitis, vascular congestion, edema, exudate, and an eczematoid dermatitis. Severe reactions are manifested by hypotension with progressive respiratory depression. Coma and death have been reported in the very young.
OVERDOSAGE
Systemic atropine toxicity is manifested by flushing and dryness of the skin (a rash may be present in pediatric patients), blurred vision, a rapid and irregular pulse, fever, abdominal distension in infants, mental aberration (hallucinosis) and loss of neuromuscular coordination.
Atropine poisoning, although distressing, is rarely fatal, even with large doses of atropine, and is self-limited if the cause is recognized and the atropine medication is discontinued. In severe intoxication, physostigmine salicylate may be administered parenterally to provide more prompt relief of the intoxication. Give physostigmine salicylate as 1-5 mL IV of dilution containing 1 mg in 5 mL of saline. The smaller dose is for pediatric patients, and injection should take not less than two minutes. EKG control is advisable. Dosage can be repeated every five minutes up to a total dose of 2 mg in pediatric patients and 6 mg in adults every 30 minutes. Physostigmine is contraindicated in hypotensive reactions. Atropine (1 mg) should be available for immediate injection if physostigmine causes bradycardia, convulsions or bronchoconstriction. In pediatric patients, the body surface must be kept moist.
Use extreme caution when employing short-acting barbiturates to control excitement.
DOSAGE AND ADMINISTRATION
ATROPINE SULFATE SOLUTION: 1 or 2 drops in the eye(s) three times a day or as directed by a physician.
FOR OPHTHALMIC USE ONLY.
| ATROPINE SULFATE
atropine sulfate solution |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Bausch & Lomb Incorporated (196603781) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Bausch & Lomb Incorporated | 079587625 | MANUFACTURE(24208-750) | |