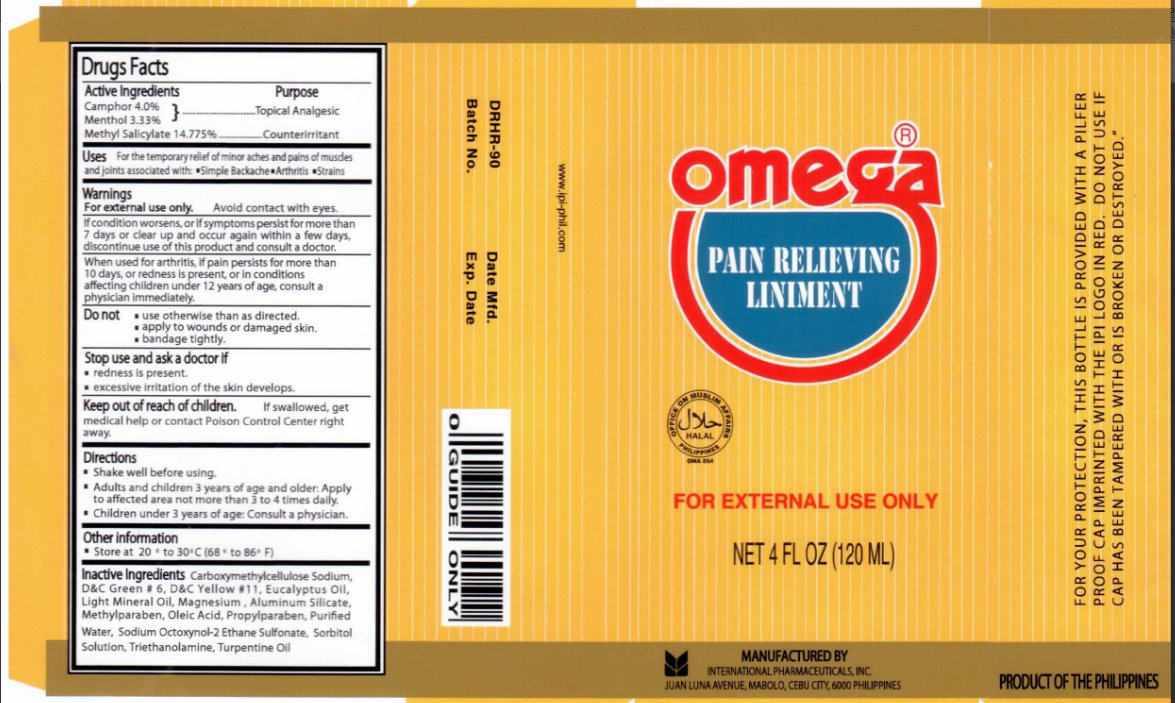
OMEGA PAIN RELIEVING- camphor, menthol, methyl salicylate liniment
International Pharmaceuticals, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Omega Pain Relieving Liniment
Active Ingredients Purpose
Camphor 4.0 percent
............................................Topical Analgesic
Menthol 3.33 percent
Methyl Salicylate 14.775 percent .....................................Counterirritant
Uses
For the temporary relief of minor aches and pains of muscle and joints associated with:
Simple Backache
Arthritis
Strains
Warnings
For external use only. Avoid Contact with eyes.
If condition worsens, or if symptoms persist for more than
7 days or clear up and occur again within a few days,
discontinue use of this product and consult a doctor.
When used for arthritis, if pain persists for more than 10 days,
or redness is present, or in conditions affecting children under
12 years of age, consult a physician immediately.
Directions
Shake well before using.
Adults and children 3 years of age and older: Apply to affected area not more than 3 to 4 times daily.
Children under 3 years of age: Consult a physician.
Keep Out Of children Reach
If swallowed, get medical help or contact Poison Control Center right away.
Inactive Ingredients
Carboxymethylcellulose Sodium, D and C Green number 6, D and C Yellow number 11, Eucalyptus Oil, Light Mineral Oil, Magnesium, Aluminum Silicate, Methylparaben, Oleic Acid, Propylparaben, Purified Water, Sodium Octoxynol-2 Ethane Sulfonate, Sorbitol Solution, Triethanolamine, Turpentine Oil.
Manufactured By
International Pharmaceuticals, Inc.
Juan Luna Avenue, Mabolo,
Cebu City 6000 Philippines
Product Of The Philippines
Shake well before using
www.ipi-phil.com
| OMEGA PAIN RELIEVING
camphor, menthol, methyl salicylate liniment |
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
| Labeler - International Pharmaceuticals, Inc. (718692460) |
| Registrant - International Pharmaceuticals, Inc. (718692460) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| International Pharmaceuticals, Inc. | 718692460 | manufacture(58929-200) | |