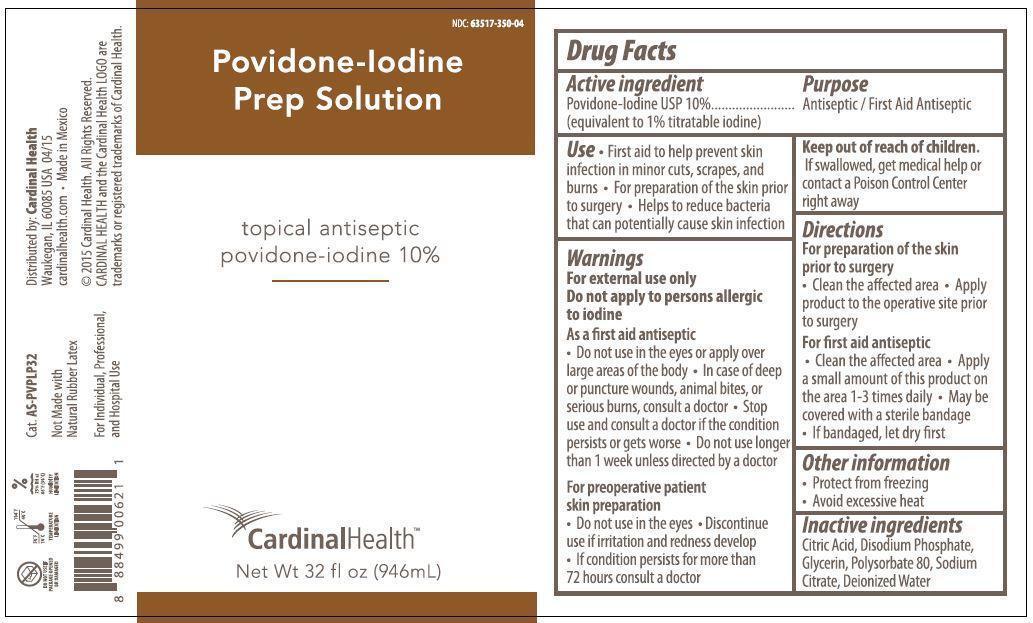
POVIDONE-IODINE PREP SOLUTION- povidone-iodine solution
Cardinal Health
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Providone- Iodine Prep Solution
topical antiseptic
Use
- First aid to help prevent skin infection in minor cuts, scrapes, and burns
- For preparation of the skin prior to surgery
- Helps to reduce bacteria that can potentially cause skin infection
Warnings
For external use only
Do not apply to persons allergic to iodine
As a first aid antiseptic
- Do not use in the eyes or apply over large areas of the body
- In case of deep or puncture wounds, animal bites, or serious burns, consult a doctor
- Stop use and consult a doctor if the condition persists or gets worse
- Do not use longer than 1 week unless directed by a doctor
For preoperative patient skin preparation
- Do not use in the eyes
- Discontinue use if irritation and redness develop
- If condition persists for more than 72 hours consult a doctor
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away
Directions
For preparation of the skin prior to surgery
- Clean the affected area
- Apply product to the operative site prior to surgery
For first aid antiseptic
- Clean the affected area
- Apply a small amount of this product on the area 1-3 times daily
- May be covered with a sterile bandage
- If bandaged, let dry first
| POVIDONE-IODINE PREP SOLUTION
povidone-iodine solution |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Cardinal Health (961027315) |
Revised: 11/2019
Document Id: 97c88a78-8024-bf00-e053-2a95a90a011b
Set id: 374587eb-aadf-4948-860a-d7e254bf96ca
Version: 3
Effective Time: 20191120
Cardinal Health