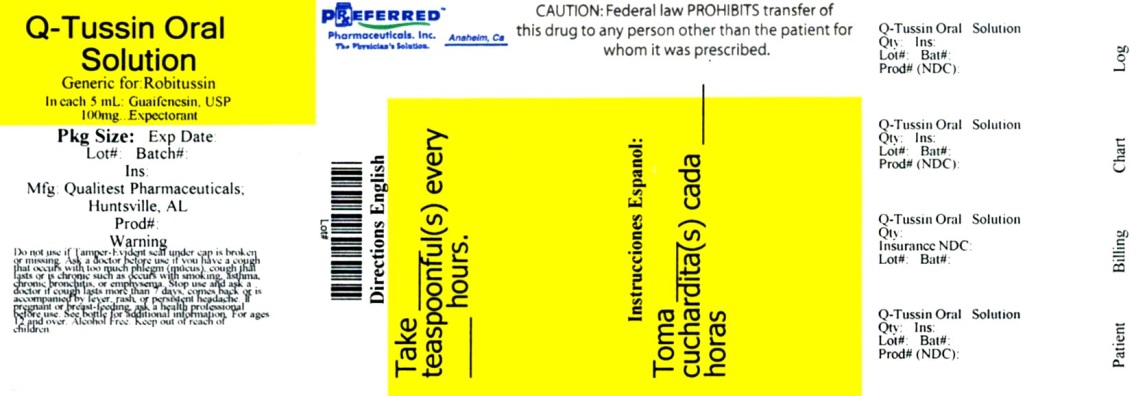
Q-TUSSIN- guaifenesin solution
Preferred Pharmaceuticals, Inc
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Q-Tussin 0857
Ask a doctor before use if you have
- •
- cough that occurs with too much phlegm (mucus)
- •
- cough that lasts or is chronic such as occurs with smoking, asthma, chronic bronchitis, or emphysema
Stop use and ask a doctor if cough lasts more than 7 days, comes back or is accompanied by fever, rash, or persistent headache. These could be signs of a serious condition.
Keep out of reach of children.
In case of overdose, get medical help or contact a Poison Control Center right away.
Directions
- •
- do not take more than 6 doses in any 24-hour period
- •
- this adult product is not intended for use in children under 12 years of age
|
age (yr) |
dose (tsp) |
|
adults and children |
2 - 4 teaspoons |
|
children under |
do not use |
Other information
- •
- each tsp contains: sodium 2 mg
- •
- store at 15° to 30°C (59° to 86°F)
- •
- dosage cup provided
You may report serious side effects to: 130 Vintage Drive, Huntsville, AL 35811.
Inactive ingredients
caramel color, FD&C red #40, flavor, glycerin, liquid glucose, purified water, saccharin sodium, sodium benzoate
| Q-TUSSIN
guaifenesin solution |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Preferred Pharmaceuticals, Inc (791119022) |
| Registrant - Preferred Pharmaceuticals, Inc (791119022) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Preferred Pharmaceuticals, Inc | 791119022 | RELABEL(68788-9081) | |