SMART SENSE ALLERGY RELIEF- fexofenadine hydrochloride tablet, film coated
Kmart Corporation
----------
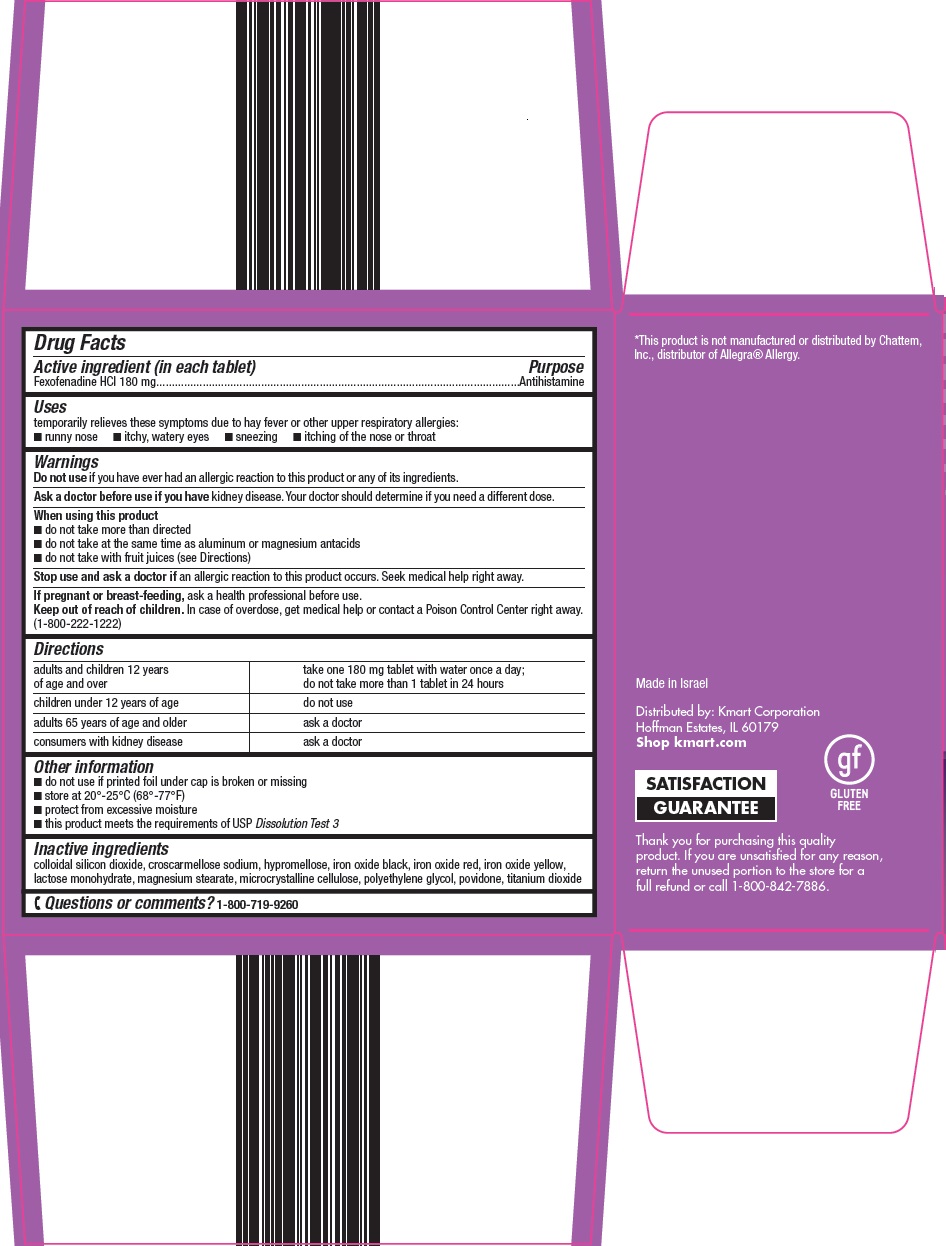
Kmart Corporation Allergy Relief Drug Facts
Uses
temporarily relieves these symptoms due to hay fever or other upper respiratory allergies:
- •
- runny nose
- •
- itchy, watery eyes
- •
- sneezing
- •
- itching of the nose or throat
Warnings
Ask a doctor before use if you have
kidney disease. Your doctor should determine if you need a different dose.
When using this product
- •
- do not take more than directed
- •
- do not take at the same time as aluminum or magnesium antacids
- •
- do not take with fruit juices (see Directions)
Directions
|
adults and children 12 years of age and over |
take one 180 mg tablet with water once a day; do not take more than 1 tablet in 24 hours |
|
children under 12 years of age |
do not use |
|
adults 65 years of age and older |
ask a doctor |
|
consumers with kidney disease |
ask a doctor |
Other information
- •
- do not use if printed foil under cap is broken or missing
- •
- store at 20°-25°C (68°-77°F)
- •
- protect from excessive moisture
- •
- this product meets the requirements of USP Dissolution Test 3
Inactive ingredients
colloidal silicon dioxide, croscarmellose sodium, hypromellose, iron oxide black, iron oxide red, iron oxide yellow, lactose monohydrate, magnesium stearate, microcrystalline cellulose, polyethylene glycol, povidone, titanium dioxide
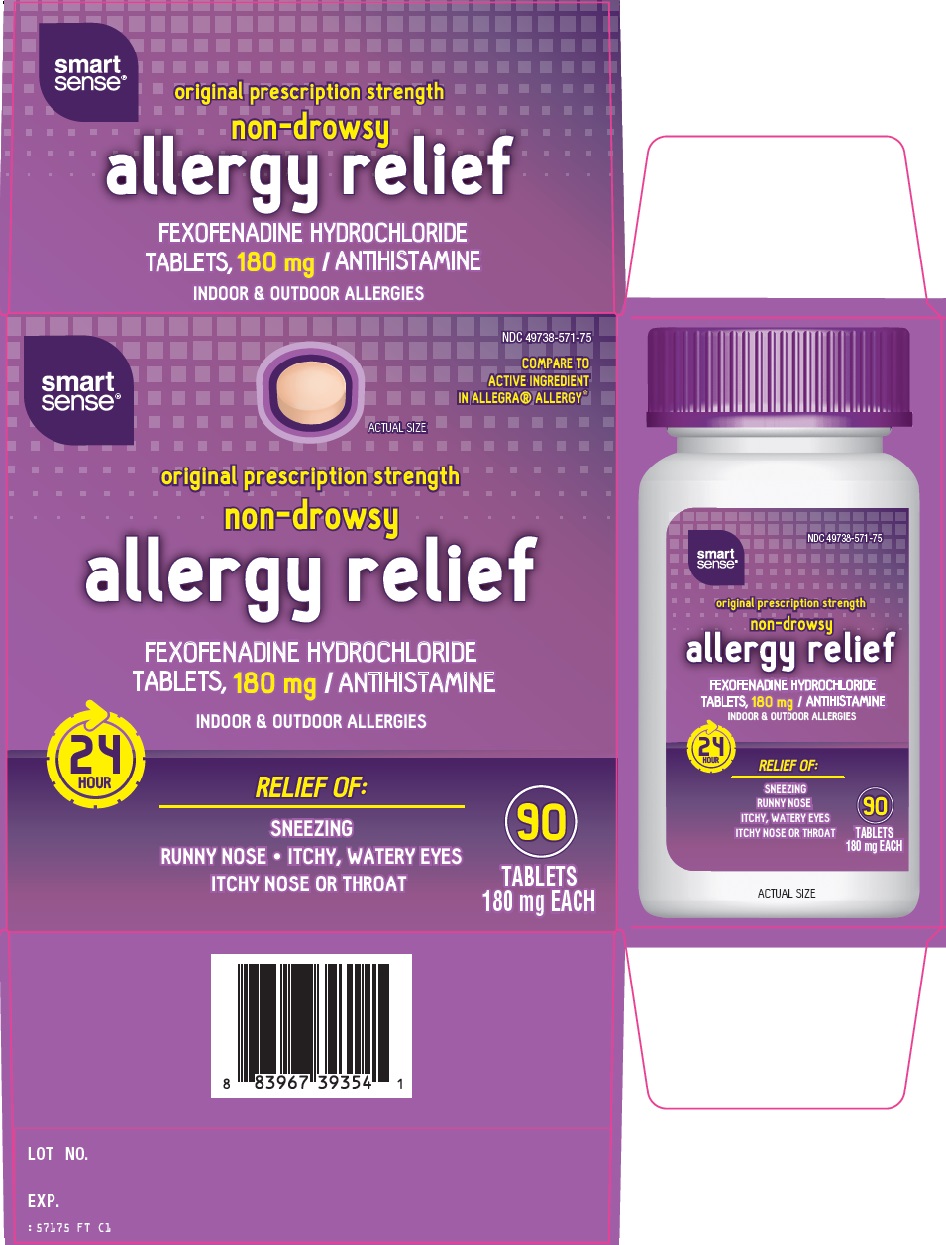
Package/Label Principal Display Panel
COMPARE TO ACTIVE INGREDIENT IN ALLEGRA® ALLERGY
ACTUAL SIZE
original prescription strength
non-drowsy
allergy relief
FEXOFENADINE HYDROCHLORIDE TABLETS, 180 mg / ANTIHISTAMINE
INDOOR & OUTDOOR ALLERGIES
24 HOUR
RELIEF OF:
SNEEZING
RUNNY NOSE - ITCHY, WATERY EYES
ITCHY NOSE OR THROAT
90 TABLETS
180 mg EACH
| SMART SENSE ALLERGY RELIEF
fexofenadine hydrochloride tablet, film coated |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Kmart Corporation (008965873) |