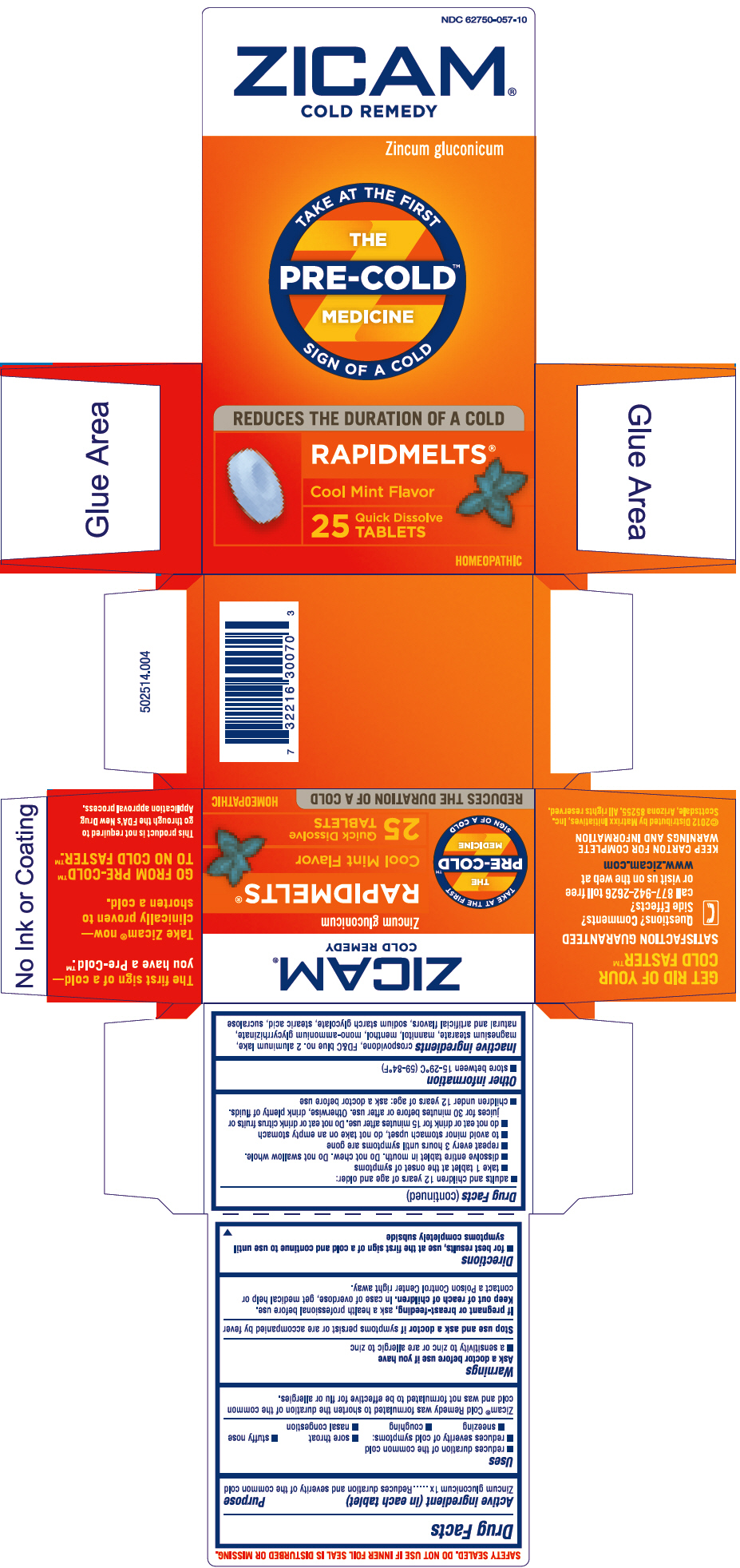
ZICAM COLD REMEDY RAPIDMELTS- zinc gluconate tablet
Matrixx Initiatives, Inc.
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
Zicam® Cold Remedy Rapidmelts®
Uses
- reduces duration of the common cold
- reduces severity of cold symptoms:
- sore throat
- stuffy nose
- sneezing
- coughing
- nasal congestion
Zicam® Cold Remedy was formulated to shorten the duration of the common cold and was not formulated to be effective for flu or allergies.
Directions
- for best results, use at the first sign of a cold and continue to use until symptoms completely subside
- adults and children 12 years of age and older:
- take 1 tablet at the onset of symptoms
- dissolve entire tablet in mouth. Do not chew. Do not swallow whole.
- repeat every 3 hours until symptoms are gone
- to avoid minor stomach upset, do not take on an empty stomach
- do not eat or drink for 15 minutes after use. Do not eat or drink citrus fruits or juices for 30 minutes before or after use. Otherwise, drink plenty of fluids.
- children under 12 years of age: ask a doctor before use
Inactive ingredients
crospovidone, FD&C blue no. 2 aluminum lake, magnesium stearate, mannitol, menthol, mono-ammonium glycyrrhizinate, natural and artificial flavors, sodium starch glycolate, stearic acid, sucralose
| ZICAM COLD REMEDY RAPIDMELTS
zinc gluconate tablet |
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Labeler - Matrixx Initiatives, Inc. (790037253) |
Revised: 10/2017
Document Id: 0502e768-b276-4b10-bd50-4c140fd82560
Set id: 361014ac-de2d-41ed-be33-169d467f53ba
Version: 2
Effective Time: 20171031
Matrixx Initiatives, Inc.