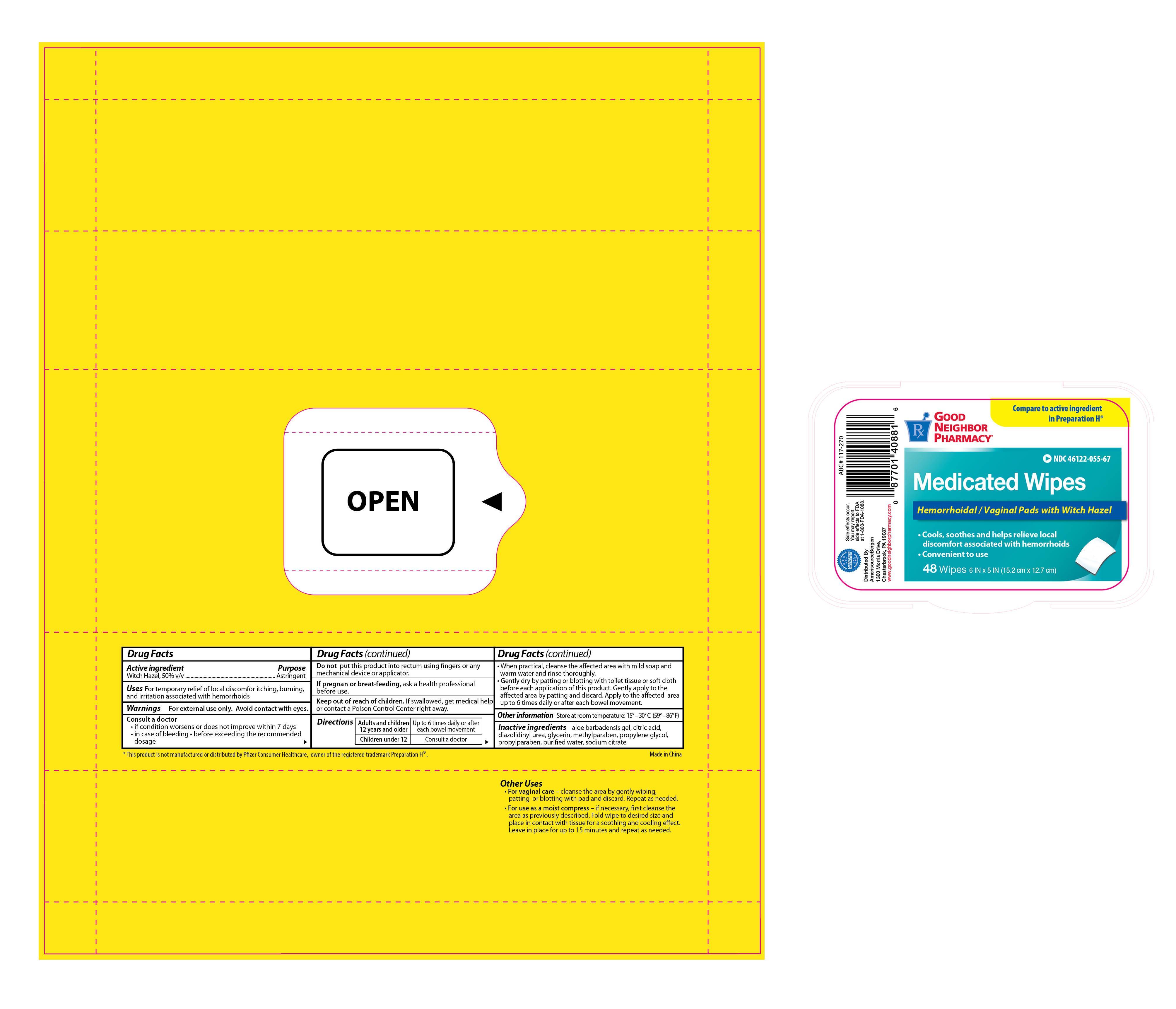
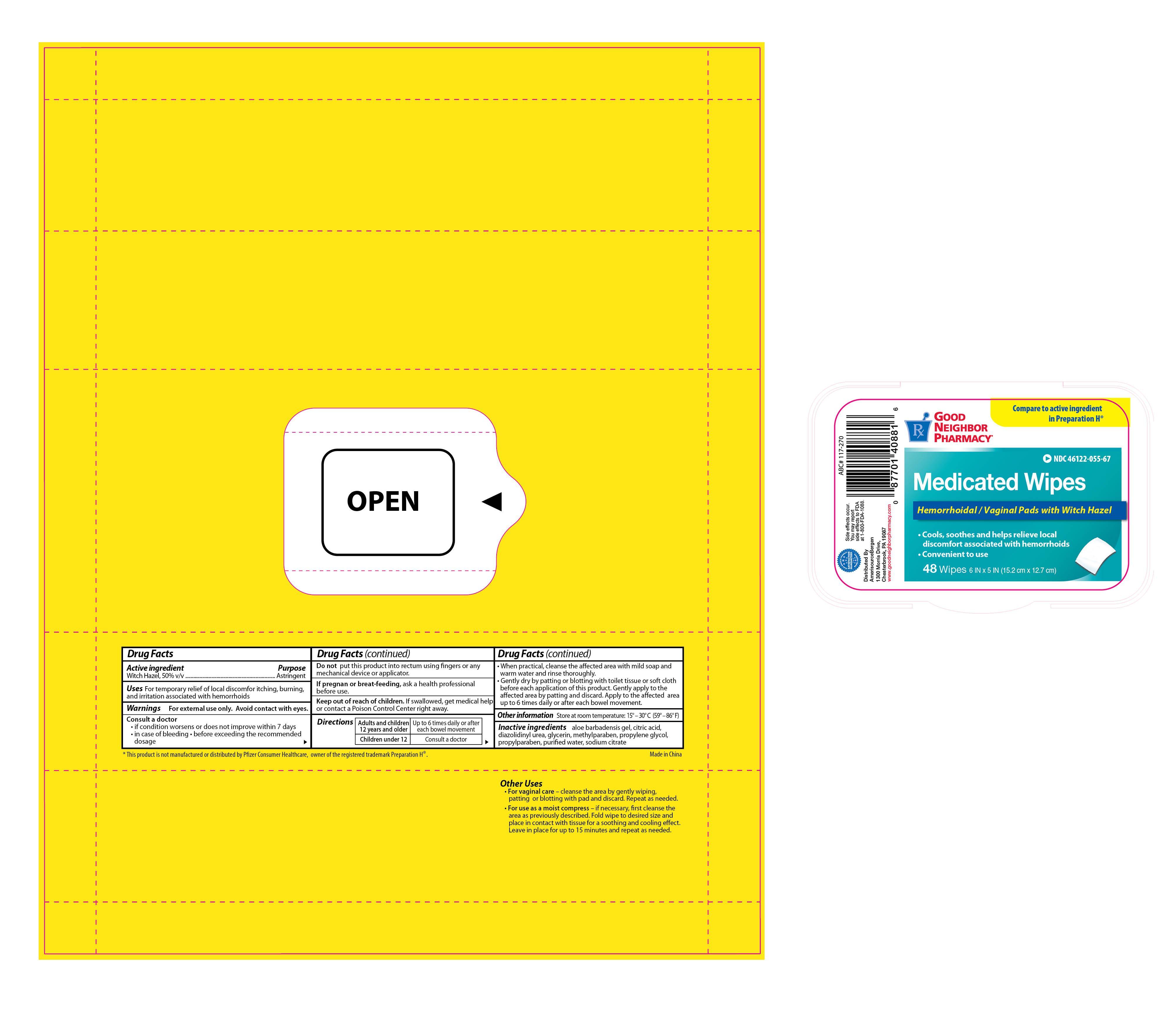
Label: GNP MEDICATED WIPES- witch hazel solution
-
Contains inactivated NDC Code(s)
NDC Code(s): 46122-055-67 - Packager: Amerisource Bergen
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated June 30, 2013
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- WARNINGS
- ASK DOCTOR
- DO NOT USE
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
-
INDICATIONS & USAGE
Directions
- Adults and children 12 years and older: up to 6 times daily or after each bowel movement
- Children under 12: consult a doctor
- When practical, cleanse the affected area with mild soap and warm water and rinse thoroughly
- Gently dry by patting or blotting with toilet tissue or soft cloth before each application of this product. Gently apply to the affected area by patting and discard. Apply to the affected area up to 6 times daily or after each bowel movement.
- STORAGE AND HANDLING
- INACTIVE INGREDIENT
- DOSAGE & ADMINISTRATION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
GNP MEDICATED WIPES
witch hazel solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:46122-055 Route of Administration TOPICAL, RECTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength WITCH HAZEL (UNII: 101I4J0U34) (WITCH HAZEL - UNII:101I4J0U34) WITCH HAZEL 0.5 mg in 1 mg Inactive Ingredients Ingredient Name Strength CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) DIAZOLIDINYL UREA (UNII: H5RIZ3MPW4) GLYCERIN (UNII: PDC6A3C0OX) METHYLPARABEN (UNII: A2I8C7HI9T) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) PROPYLPARABEN (UNII: Z8IX2SC1OH) WATER (UNII: 059QF0KO0R) SODIUM CITRATE (UNII: 1Q73Q2JULR) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:46122-055-67 48 in 1 POUCH 1 0.5 mg in 1 APPLICATOR Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part346 06/30/2013 Labeler - Amerisource Bergen (007914906)