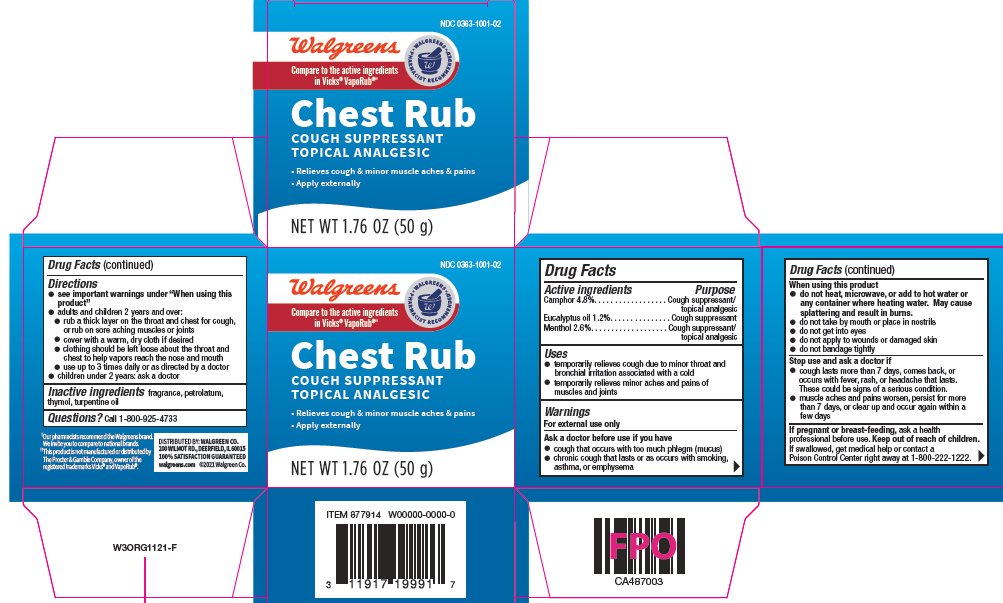
Label: WALGREENS CHEST RUB- camphor, eucalyptus oil, menthol ointment
- NDC Code(s): 0363-1001-01, 0363-1001-02
- Packager: Walgreen Company
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated April 14, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
- Purpose
- Uses
-
Warnings
For external use only
Ask a doctor before use if you have
- cough that occurs with too much phlegm (mucus)
- chronic cough that lasts or occurs with smoking, asthma, or emphysema
When using this product
- do not heat, microwave, or add to hot water or any container where heating water. May cause splattering and result in burns.
- do not take by mouth or place in nostrils
- do not get into eyes
- do not apply to wounds or damaged skin
- do not bandage tightly
- Keep out of reach of children
-
Directions
- see important warning under "When using this product"
- adults and children 2 years and over:
- rub a thick layer on the throat and chest for cough, or rub on sore aching muscles and joints
- cover with a warm, dry cloth if desired
- clothing should be left loose about the throat and chest to help vapors reach the nose and mouth
- use up to 3 times daily, or as directed by a doctor
- children under 2 years: ask a doctor
- Inactive Ingredients
- Package/Label Principal Display Panel
-
INGREDIENTS AND APPEARANCE
WALGREENS CHEST RUB
camphor, eucalyptus oil, menthol ointmentProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0363-1001 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CAMPHOR (SYNTHETIC) (UNII: 5TJD82A1ET) (CAMPHOR (SYNTHETIC) - UNII:5TJD82A1ET) CAMPHOR (SYNTHETIC) 48 mg in 1 g EUCALYPTUS OIL (UNII: 2R04ONI662) (EUCALYPTUS OIL - UNII:2R04ONI662) EUCALYPTUS OIL 12 mg in 1 g MENTHOL, UNSPECIFIED FORM (UNII: L7T10EIP3A) (MENTHOL, UNSPECIFIED FORM - UNII:L7T10EIP3A) MENTHOL, UNSPECIFIED FORM 26 mg in 1 g Inactive Ingredients Ingredient Name Strength PETROLATUM (UNII: 4T6H12BN9U) THYMOL (UNII: 3J50XA376E) TURPENTINE OIL (UNII: C5H0QJ6V7F) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0363-1001-01 1 in 1 CARTON 03/01/2015 1 100 g in 1 JAR; Type 0: Not a Combination Product 2 NDC:0363-1001-02 1 in 1 CARTON 07/01/2018 2 50 g in 1 JAR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part341 03/01/2015 Labeler - Walgreen Company (008965063)