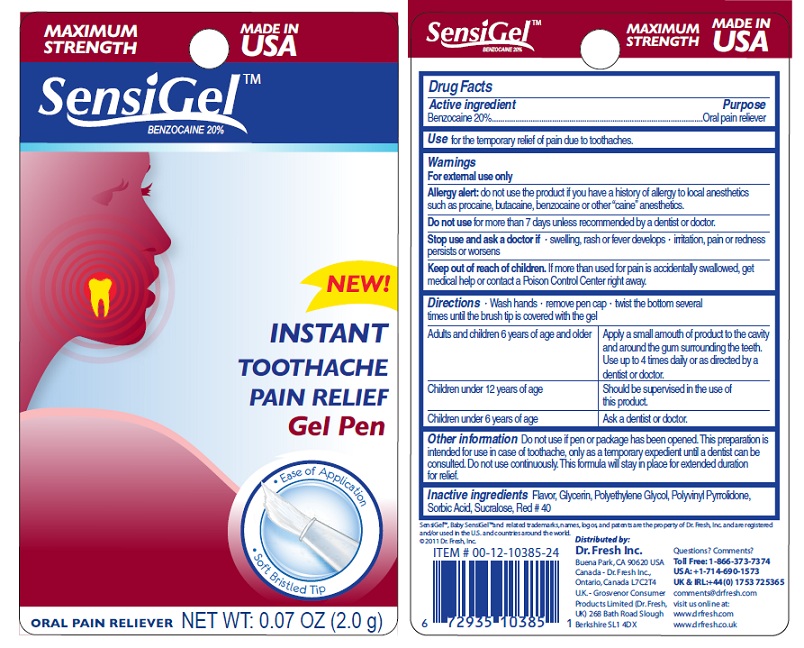
SENSIGEL- benzocaine gel
Dr. Fresh, LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
MAXIMUM STRENGTH SENSIGEL
Warnings
For external use only.
Allergy Alert: do not use the product if you havd a history of allergy to local anesthetics such as procaine, butacaine, benzocaine or other "caine" anesthetics.
Directions
Wash hands - remove pen cap - twist the bottom several times until the brush tip is covered with the gel.
Adults and children 6 years of age and older: Apply a small amount of product to the cavity and around the gum surrounding the teeth. Use up to 4 times daily or as directed by a dentist or doctor. Children under 12 years of age - Should be supervised in the use of this product. Children under 6 years of age: Ask a dentist or doctor.
Other Information
Do not use if pen or package has been opened. This preparation is intended for use in case of toothache, only as a temporary expedient until a dentist can b consulted. Do not use continuously. This formula will stay in place for an extended duration for relief.
| SENSIGEL
benzocaine gel |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - Dr. Fresh, LLC (117376803) |