Label: MEGACE ES- megestrol acetate suspension
-
Contains inactivated NDC Code(s)
NDC Code(s): 16590-254-33 - Packager: Stat Rx USA
- This is a repackaged label.
- Source NDC Code(s): 49884-949
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated October 27, 2009
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
DESCRIPTION
Megace ® ES (megestrol acetate) oral suspension contains megestrol acetate, a synthetic derivative of the naturally occurring steroid hormone, progesterone. Megestrol acetate is a white, crystalline solid chemically designated as 17-Hydroxy-6-methylpregna-4,6-diene-3,20-dione acetate. Solubility at 37° C in water is 2 mcg per mL, solubility in plasma is 24 mcg per mL. Its molecular weight is 384.52.
The chemical formula is C24H32O4 and the structural formula is represented as follows:
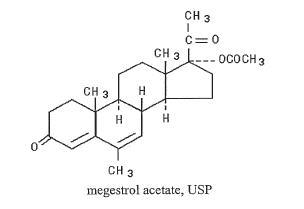
Megace ® ES (megestrol acetate) is a concentrated formula supplied as an oral suspension containing 125 mg of megestrol acetate per mL.
Megace ® ES (megestrol acetate) oral suspension contains the following inactive ingredients: alcohol (max 0.06% v/v from flavor), artificial lime flavor, citric acid monohydrate, docusate sodium, hydroxypropyl methylcellulose (hypromellose), natural and artificial lemon flavor, purified water, sodium benzoate, sodium citrate dihydrate, and sucrose.
-
CLINICAL PHARMACOLOGY
CLINICAL PHARMACOLOGY
There are several analytical methods used to estimate megestrol acetate plasma concentrations, including gas chromatography-mass fragmentography (GC-MF), high pressure liquid chromatography (HPLC) and radioimmunoassay (RIA). The GC-MF and HPLC methods are specific for megestrol acetate and yield equivalent concentrations. The RIA method reacts to megestrol acetate metabolites and is, therefore, non-specific and indicates higher concentrations than the GC-MF and HPLC methods. Plasma concentrations are dependent, not only on the method used, but also on intestinal and hepatic inactivation of the drug, which may be affected by factors such as intestinal tract motility, intestinal bacteria, antibiotics administered, body weight, diet and liver function.
Mechanism of ActionSeveral investigators have reported on the appetite enhancing property of megestrol acetate and its possible use in cachexia. The precise mechanism by which megestrol acetate produces effects in anorexia and cachexia is unknown at the present time.
Pharmacokinetic Properties:Plasma concentrations of megestrol acetate after administration of 625 mg (125 mg/mL) of Megace® ES oral suspension are equivalent under fed conditions to 800 mg (40 mg/mL) of megestrol acetate oral suspension (see figure below).
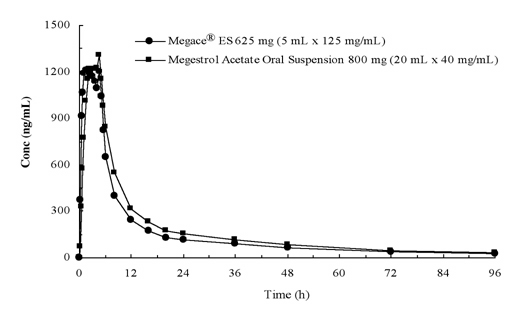
In order to characterize the dose proportionality of Megace® ES, pharmacokinetic studies across a range of doses were conducted when administered under fasting and fed conditions. Pharmacokinetics of megestrol was linear in the dosing range between 150 mg and 675 mg after Megace® ES administration regardless of meal condition. The Cmax and AUC under a high fat meal were increased by 48% and 36%, respectively, compared to those under the fasting after 625 mg Megace® ES administration (Table 1). However, a high fat meal significantly increased AUC and Cmax of megestrol to 2-fold and 7-fold, respectively, compared to those under fasting condition after administration of 800 mg in the original formulation. There was no difference in safety following administration in the fed state, therefore Megace® ES could be taken without regard to meals.
Table 1 - Pharmacokinetic Studies Conducted with Megace® ES Amount Dosed 150 mg 250 mg 375 mg 450 mg 575 mg 625 mg 675 mg 800 mg* Dose 5 mL 5 mL 5 mL 5 mL 5 mL 5 mL 5 mL 20 mL Fast Fed Fast Fed Fast Fed Fast Fed Fast Fed Fast Fed Fast Fed Fast Fed Cmax (ng/mL) 412 379 647 588 810 958 955 1079 - 1421 1133 1618 1044 1616 187 1364 AUC0-∞ (ng∙h/mL) 3058 3889 5194 6328 7238 12193 9483 11800 - 14743 12095 16268 11879 17029 8942 18625 Tmax (h) 1.74 3.80 1.58 3.38 1.56 3.42 1.74 3.16 - 3.75 1.72 2.91 1.96 2.76 5.89 3.85 Plasma steady state pharmacokinetics of megestrol acetate were evaluated in 10 adult, cachectic male patients with acquired immunodeficiency syndrome (AIDS) and an involuntary weight loss greater than 10% of baseline. Patients received single oral doses of 800 mg/day of megestrol acetate oral suspension for 21 days. Plasma concentration data obtained on day 21 were evaluated for up to 48 hours past the last dose.
Mean (±1SD) peak plasma concentration (Cmax) of megestrol acetate was 753 (±539) ng/mL. Mean area under the concentration time-curve (AUC) was 10476 (±7788) ng x hr/mL. Median Tmax value was five hours. Seven of 10 patients gained weight in three weeks.
Additionally, 24 adult, asymptomatic HIV seropositive male subjects were dosed once daily with 750 mg of megestrol acetate oral suspension. The treatment was administered for 14 days. Mean Cmax and AUC values were 490 (±238) ng/mL and 6779 (±3048) hr x ng/mL, respectively. The median Tmax value was three hours. The mean Cmax value was 202 (±101) ng/mL. The mean % of fluctuation value was 107 (±40).
MetabolismMegestrol acetate metabolites which were identified in urine constituted 5% to 8% of the dose administered. Respiratory excretion as labeled carbon dioxide and fat storage may have accounted for at least part of the radioactivity not found in urine and feces.
EliminationThe major route of drug elimination in humans is urine. When radiolabeled megestrol acetate was administered to humans in doses of 4 to 90 mg, the urinary excretion within 10 days ranged from 56.5% to 78.4% (mean 66.4%) and fecal excretion ranged from 7.7% to 30.3% (mean 19.8%). The total recovered radioactivity varied between 83.1% and 94.7% (mean 86.2%).
Special PopulationsThe pharmacokinetics of megestrol acetate has not been studied in any special populations.
ANIMAL PHARMACOLOGY AND/OR TOXICOLOGYLong-term treatment with Megace ® ES (megestrol acetate) may increase the risk of respiratory infections. A trend toward increased frequency of respiratory infections, decreased lymphocyte counts and increased neutrophil counts was observed in a two-year chronic toxicity/carcinogenicity study of megestrol acetate conducted in rats.
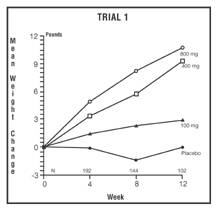
DESCRIPTION OF CLINICAL STUDIESMegestrol acetate oral suspension at a dose of 800 mg/20 mL is equivalent to 625 mg/5 mL of Megace ® ES. The clinical efficacy of megestrol acetate oral suspension was assessed in two clinical trials. One was a multicenter, randomized, double-blind, placebo-controlled study comparing megestrol acetate (MA) at doses of 100 mg, 400 mg, and 800 mg per day versus placebo in AIDS patients with anorexia/cachexia and significant weight loss. Of the 270 patients entered on study, 195 met all inclusion/exclusion criteria, had at least two additional post baseline weight measurements over a 12 week period or had one post baseline weight measurement but dropped out for therapeutic failure. The percent of patients gaining five or more pounds at maximum weight gain in 12 study weeks was statistically significantly greater for the 800 mg (64%) and 400 mg (57%) MA-treated groups than for the placebo group (24%). Mean weight increased from baseline to last evaluation in 12 study weeks in the 800 mg MA-treated group by 7.8 pounds, the 400 mg MA group by 4.2 pounds, the 100 mg MA group by 1.9 pounds and decreased in the placebo group by 1.6 pounds. Mean weight changes at 4, 8 and 12 weeks for patients evaluable for efficacy in the two clinical trials are shown graphically. Changes in body composition during the 12 study weeks as measured by bioelectrical impedance analysis showed increases in non-water body weight in the MA-treated groups (see clinical studies table). In addition, edema developed or worsened in only 3 patients.
Greater percentages of MA-treated patients in the 800 mg group (89%), the 400 mg group (68%) and the 100 mg group (72%), than in the placebo group (50%), showed an improvement in appetite at last evaluation during the 12 study weeks. A statistically significant difference was observed between the 800 mg MA-treated group and the placebo group in the change in caloric intake from baseline to time of maximum weight change. Patients were asked to assess weight change, appetite, appearance, and overall perception of well-being in a 9 question survey. At maximum weight change only the 800 mg MA-treated group gave responses that were statistically significantly more favorable to all questions when compared to the placebo-treated group. A dose response was noted in the survey with positive responses correlating with higher dose for all questions.
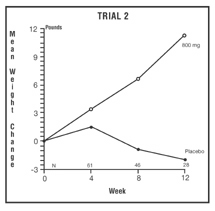
The second trial was a multicenter, randomized, double-blind, placebo-controlled study comparing megestrol acetate 800 mg/day versus placebo in AIDS patients with anorexia/cachexia and significant weight loss. Of the 100 patients entered on study, 65 met all inclusion/exclusion criteria, had at least two additional post baseline weight measurements over a 12 week period or had one post baseline weight measurement but dropped out for therapeutic failure. Patients in the 800 mg MA-treated group had a statistically significantly larger increase in mean maximum weight change than patients in the placebo group. From baseline to study week 12, mean weight increased by 11.2 pounds in the MA-treated group and decreased 2.1 pounds in the placebo group. Changes in body composition as measured by bioelectrical impedance analysis showed increases in non-water weight in the MA-treated group (see clinical studies table). No edema was reported in the MA-treated group. A greater percentage of MA-treated patients (67%) than placebo-treated patients (38%) showed an improvement in appetite at last evaluation during the 12 study weeks; this difference was statistically significant. There were no statistically significant differences between treatment groups in mean caloric change or in daily caloric intake at time to maximum weight change. In the same 9 question survey referenced in the first trial, patients’ assessments of weight change, appetite, appearance, and overall perception of well-being showed increases in mean scores in MA-treated patients as compared to the placebo group.
In both trials, patients tolerated the drug well and no statistically significant differences were seen between the treatment groups with regard to laboratory abnormalities, new opportunistic infections, lymphocyte counts, T4 counts, T8 counts, or skin reactivity tests (see ADVERSEREACTIONS section).
Megestrol Acetate Oral Suspension Clinical Efficacy Trials Trial 1 Trial 2 Study Accrual Dates Study Accrual Dates 11/88 to 12/90 5/89 to 4/91 Megestrol Acetate, mg/day 0 100 400 800 0 800 Entered Patients 38 82 75 75 48 52 Evaluable Patients 28 61 53 53 29 36 Mean Change in Weight (lb.) Baseline to 12 Weeks 0.0 2.9 9.3 10.7 -2.1 11.2 % Patients ≥ 5 Pound Gain At Last Evaluation in 12 weeks 21 44 57 64 28 47 Mean Changes in Body Composition: Fat Body Mass (lb.) 0.0 2.2 2.9 5.5 1.5 5.7 Lean Body Mass (lb.) -1.7 -0.3 1.5 2.5 -1.6 -0.6 Water (liters) -1.3 -0.3 0.0 0.0 -0.1 -0.1 % Patients With Improved Appetite: At Time of Maximum Weight Change 50 72 72 93 48 69 At Last Evaluation in 12 Weeks 50 72 68 89 38 67 Mean Change in Daily Caloric Intake: Baseline to Time of Maximum Weight Change -107 326 308 646 30 464 *Based on bioelectrical impedance analysis determinations at last evaluation in 12 weeks Presented below are the results of mean weight changes for patients evaluable for efficacy in trials 1 and 2.
- INDICATIONS & USAGE
- CONTRAINDICATIONS
-
WARNINGS AND PRECAUTIONS
WARNINGS
Megestrol acetate may cause fetal harm when administered to a pregnant woman. For animal data on fetal effects, (see PRECAUTIONS:Impairment of Fertility section). There are no adequate and well-controlled studies in pregnant women. If this drug is used during pregnancy, or if the patient becomes pregnant while taking (receiving) this drug, the patient should be apprised of the potential hazard to the fetus. Women of childbearing potential should be advised to avoid becoming pregnant.
Megestrol acetate is not intended for prophylactic use to avoid weight loss.
(See also PRECAUTIONS: Carcinogenesis, Mutagenesis, and Impairment of Fertility section).
The glucocorticoid activity of megestrol acetate oral suspension has not been fully evaluated. Clinical cases of new onset diabetes mellitus, exacerbation of pre-existing diabetes mellitus, and overt Cushing’s Syndrome have been reported in association with the chronic use of megestrol acetate. In addition, clinical cases of adrenal insufficiency have been observed in patients receiving or being withdrawn from chronic megestrol acetate therapy in the stressed and non-stressed state. Furthermore, adrenocorticotropin (ACTH) stimulation testing has revealed the frequent occurrence of asymptomatic pituitary-adrenal suppression in patients treated with chronic megestrol acetate therapy. Therefore, the possibility of adrenal insufficiency should be considered in any patient receiving or being withdrawn from chronic Megace ® ES therapy who presents with symptoms and/or signs suggestive of hypoadrenalism (e.g., hypotension, nausea, vomiting, dizziness, or weakness) in either the stressed or non-stressed state. Laboratory evaluation for adrenal insufficiency and consideration of replacement or stress doses of a rapidly acting glucocorticoid are strongly recommended in such patients. Failure to recognize inhibition of the hypothalamic-pituitary-adrenal axis may result in death. Finally, in patients who are receiving or being withdrawn from chronic Megace® ES therapy, consideration should be given to the use of empiric therapy with stress doses of a rapidly acting glucocorticoid during stress or serious intercurrent illness (e.g., surgery, infection).
PRECAUTIONS
GENERAL
Therapy with Megace ® ES (megestrol acetate) oral suspension for weight loss should only be instituted after treatable causes of weight loss are sought and addressed. These treatable causes include possible malignancies, systemic infections, gastrointestinal disorders affecting absorption, endocrine disease and renal or psychiatric diseases.
Effects on HIV viral replication have not been determined.
Use with caution in patients with a history of thromboembolic disease.
Use in DiabeticsExacerbation of pre-existing diabetes with increased insulin requirements have been reported in association with the use of megestrol acetate.
-
INFORMATION FOR PATIENTS
Information for Patients
Patients using Megace ® ES (megestrol acetate) should receive the following instructions:
- This medication is to be used as directed by the physician.
- Megace® ES (625 mg/5 mL) does not contain the same amount of megestrol acetate as Megace® oral suspension or any of the other megestrol acetate oral suspensions. Megace® ES contains 625 mg of megestrol acetate per 5 mL whereas Megace® oral suspension and other megestrol acetate oral suspensions contain 800 mg per 20 mL.
- The prescriber should inform the patient about the product differences to avoid overdosing or underdosing of megestrol acetate. The recommended adult dosage of Megace® ES is one teaspoon (5 mL) once a day. Please see table in DOSAGE AND ADMINISTRATION section.
- Report any adverse reaction experiences while taking this medication.
- Use contraception while taking this medication if you are a woman capable of becoming pregnant.
- Notify your physician if you become pregnant while taking this medication.
Pharmacokinetic studies show that there are no significant alterations in pharmacokinetic parameters of zidovudine or rifabutin to warrant dosage adjustment when megestrol acetate is administered with these drugs. A pharmacokinetic study demonstrated that coadministration of megestrol acetate and indinavir results in a significant decrease in the pharmacokinetic parameters (~36% for C max and ~28% for AUC) of indinavir. Administration of a higher dose of indinavir should be considered when coadministering with megestrol acetate. The effects of indinavir, zidovudine or rifabutin on the pharmacokinetics of megestrol acetate were not studied.
Carcinogenesis and Mutagenesis and Impairment of FertilityData on carcinogenesis were obtained from studies conducted in dogs, monkeys and rats treated with megestrol acetate at doses 53.2, 26.6 and 1.3 times lower than the proposed dose (13.3 mg/kg/day) for humans. No males were used in the dog and monkey studies. In female beagles, megestrol acetate (0.01, 0.1 or 0.25 mg/kg/day) administered for up to 7 years induced both benign and malignant tumors of the breast. In female monkeys, no tumors were found following 10 years of treatment with 0.01, 0.1 or 0.5 mg/kg/day megestrol acetate. Pituitary tumors were observed in female rats treated with 3.9 or 10 mg/kg/day of megestrol acetate for 2 years. The relationship of these tumors in rats and dogs to humans is unknown but should be considered in assessing the risk-to-benefit ratio when prescribing Megace ® ES (megestrol acetate) oral suspension and in surveillance of patients on therapy. (See WARNINGS section).
MutagenesisNo mutagenesis data are currently available.
Impairment of FertilityPerinatal/postnatal (segment III) toxicity studies were performed in rats at doses (0.05 to 12.5 mg/kg) less than that indicated for humans (13.3 mg/kg); in these low dose studies, the reproductive capability of male offspring of megestrol acetate-treated females was impaired. Similar results were obtained in dogs. Pregnant rats treated with megestrol acetate showed a reduction in fetal weight and number of live births, and feminization of male fetuses. No toxicity data are currently available on male reproduction (spermatogenesis).
PregnancyPregnancy Category X. (See WARNINGS and PRECAUTIONS: Impairment of Fertility sections). No adequate animal teratology information is available at clinically relevant doses.
Nursing MothersBecause of the potential for adverse effects on the newborn, nursing should be discontinued if Megace ® ES (megestrol acetate) oral suspension is required.
Use in HIV Infected WomenAlthough megestrol acetate has been used extensively in women for the treatment of endometrial and breast cancers, its use in HIV infected women has been limited.
All 10 women in the clinical trials reported breakthrough bleeding.
Pediatric UseSafety and effectiveness in pediatric patients have not been established.
Geriatric UseClinical studies of megestrol acetate oral suspension in the treatment of anorexia, cachexia, or an unexplained, significant weight loss in patients with AIDS did not include sufficient numbers of patients aged 65 years and older to determine whether they respond differently than younger patients. Other reported clinical experience has not identified differences in responses between elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
Megestrol acetate is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function.
-
ADVERSE REACTIONS
ADVERSE REACTIONS
Adverse events which occurred in at least 5% of patients in any arm of the two clinical efficacy trials and the open trial are listed below by treatment group. All patients listed had at least one post baseline visit during the 12 study weeks. These adverse events should be considered by the physician when prescribing Megace ® ES (megestrol acetate) oral suspension.
ADVERSE EVENTS% of Patients Reporting Open Trial 1 (N=236) Trial 2 (N=87) Label Trial Megestrol Acetate, Placebo Placebo mg/day 0 100 400 800 0 800 1200 No. of Patients N=34 N=68 N=69 N=65 N=38 N=49 N=176 Diarrhea 15 13 8 15 8 6 10 Impotence 3 4 6 14 0 4 7 Rash 9 9 4 12 3 2 6 Flatulence 9 0 1 9 3 10 6 Hypertension 0 0 0 8 0 0 4 Asthenia 3 2 3 6 8 4 5 Insomnia 0 3 4 6 0 0 1 Nausea 9 4 0 5 3 4 5 Anemia 6 3 3 5 0 0 0 Fever 3 6 4 5 3 2 1 Libido Decreased 3 4 0 5 0 2 1 Dyspepsia 0 0 3 3 5 4 2 Hyperglycemia 3 0 6 3 0 0 3 Headache 6 10 1 3 3 0 3 Pain 6 0 0 2 5 6 4 Vomiting 9 3 0 2 3 6 4 Pneumonia 6 2 0 2 3 0 1 Urinary Frequency 0 0 1 2 5 2 1 Adverse events which occurred in 1% to 3% of all patients enrolled in the two clinical efficacy trials with at least one follow-up visit during the first 12 weeks of the study are listed below by body system. Adverse events occurring less than 1% are not included. There were no significant differences between incidence of these events in patients treated with megestrol acetate and patients treated with placebo.
Body as a Whole - abdominal pain, chest pain, infection, moniliasis and sarcoma
Cardiovascular System - cardiomyopathy and palpitation
Digestive System - constipation, dry mouth, hepatomegaly, increased salivation and oral moniliasis
Hemic and Lymphatic System - leukopenia
Metabolic and Nutritional - LDH increased, edema and peripheral edema
Nervous System - paresthesia, confusion, convulsion, depression, neuropathy, hypesthesia and abnormal thinking
Respiratory System - dyspnea, cough, pharyngitis and lung disorder
Skin and Appendages - alopecia, herpes, pruritus, vesiculobullous rash, sweating and skin disorder
Urogenital System - albuminuria, urinary incontinence, urinary tract infection and gynecomastia
PostmarketingPostmarketing reports associated with megestrol acetate oral suspension include thromboembolic phenomena including thrombophlebitis, deep vein thrombosis, and pulmonary embolism; and glucose intolerance (see WARNINGS and PRECAUTIONS sections).
-
OVERDOSAGE
OVERDOSAGE
No serious unexpected side effects have resulted from studies involving megestrol acetate oral suspension administered in dosages as high as 1200 mg/day. Megestrol acetate has not been tested for dialyzability; however, due to its low solubility it is postulated that dialysis would not be an effective means of treating overdose.
-
DOSAGE & ADMINISTRATION
DOSAGE AND ADMINISTRATION
The recommended adult initial dosage of Megace ® ES (megestrol acetate) oral suspension is 625 mg/day (5 mL/day or one teaspoon daily). Please refer to the table below for correct dosing and administration. Shake container well before using.
PRODUCT DIFFERENCES Megace® ES Oral Suspension Megace® and other megestrol acetate oral suspensions mg/mL 125 mg/mL 40 mg/mL Recommended Daily Dose 625 mg 800 mg Daily Volume Intake 5 mL (teaspoon) 20 mL (dosing cup) Formulation Concentrated formula Regular formula In clinical trials evaluating different dose schedules, daily doses of 400 and 800 mg/day of megestrol acetate oral suspension (800 mg/20 mL equivalent to 625 mg/5 mL of Megace ® ES formula) were found to be clinically effective.
-
HOW SUPPLIED
HOW SUPPLIED
Megace ® ES (megestrol acetate) oral suspension is a concentrated formula available as a milky white, lemon-lime flavored oral suspension containing 125 mg of megestrol acetate per mL.
STORAGENDC 49884-949-69 Bottles of 150 mL (5 fl. oz.) Store Megace ® ES (megestrol acetate) oral suspension between 15º to 25º C (59º to 77º F) and dispense in a tight container. Protect from heat.
SPECIAL HANDLINGThere is no threshold limit value established by OSHA, NIOSH, or ACGIH.
Exposure or overdose at levels approaching recommended dosing levels could result in side effects described above (see WARNINGS and ADVERSE REACTIONS sections). Women at risk of pregnancy should avoid such exposure.
PAR PHARMACEUTICAL COMPANIES, INC.
Revised: 05/08 OS939-51-1-03 Megace ® is a registered trademark of Bristol-Myers Squibb Company licensed to Par Pharmaceutical, Inc.
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
MEGACE ES
megestrol acetate suspensionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:16590-254(NDC:49884-949) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MEGESTROL ACETATE (UNII: TJ2M0FR8ES) (MEGESTROL - UNII:EA6LD1M70M) MEGESTROL ACETATE 125 mg in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:16590-254-33 150 mL in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA021778 06/08/2005 Labeler - Stat Rx USA (786036330)


