NON ASPIRIN EXTRA STRENGTH- acetaminophen tablet
Chain Drug Consortium
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
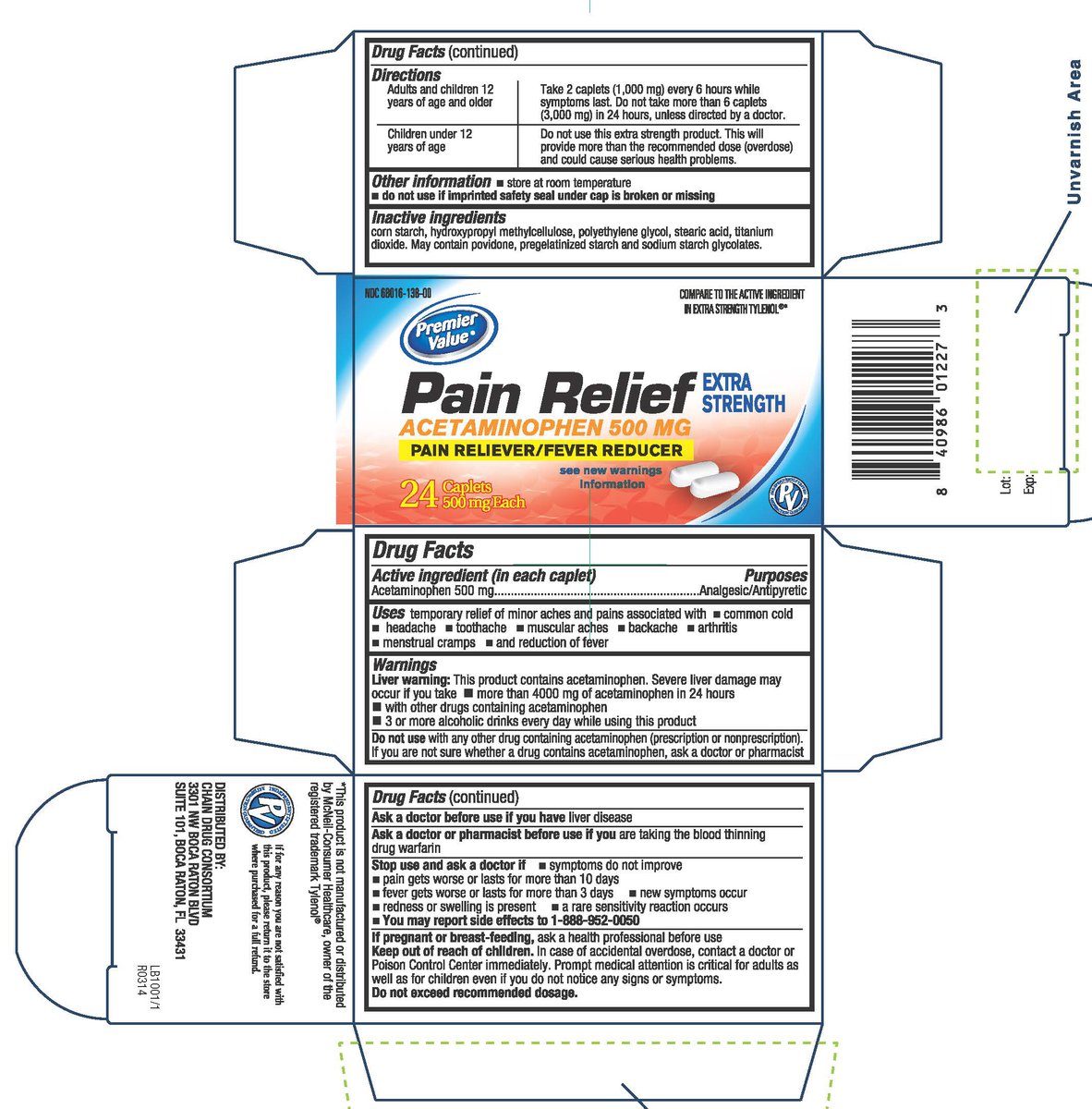
Drug Facts
Uses
temporary relief of minor aches and pains associated with common cold headache toothache
muscular aches backache arthritis menstrual cramps and reduction of fever
Warnings
Liver warning: This product contains acetaminophen. Severe liver damage may occur if you takemore than 4000mg of acetaminophen in 24 hours with other drugs containing acetaminophen 3 or more alcoholic drinks every day while using this product
Do Not Use
with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist
Ask Doctor doctor before use if you have
liver disease
Ask Doctor/Pharmacist before use if you
are taking the blood thinning drug warfarin
Keep Out of Reach of Children
In case of accidental overdose, contact a doctor or Poison Control Center immediately. Prompt medical attention is critical for adults as well as for children even if you do not notice any signs or symptoms. Do not exceed recommended dosage.
Directions
Adults and children 12 years of age and older: Take 2 caplets (1,000 mg) every 6 hours while symptoms last. Do not take more than 6 caplets (3,000 mg) in 24 hours, unless directed by a doctor.
Children under 12 years of age: Do not use this extra strength product. This will provide more than the recommended dose (overdose) and could cause serious health problems.
| NON ASPIRIN
EXTRA STRENGTH
acetaminophen tablet |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Labeler - Chain Drug Consortium (101668460) |
| Registrant - Chain Drug Consortium (101668460) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| A&Z Pharmaceutical, Inc. | 926820705 | label(68016-139) , manufacture(68016-139) , pack(68016-139) , relabel(68016-139) , repack(68016-139) | |