MULTI VITAMIN DROPS WITH FLUORIDE- vitamin a palmitate, ascorbic acid, cholecalciferol, alpha-tocopherol succinate, d-thiamine hydrochloride, riboflavin 5-phosphate sodium, cyanocobalamin, niacinamide, pyridoxine hydrochloride and sodium fluoride solution/ drops
Seton Pharmaceuticals
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
Multi-Vitamin Drops with Fluoride
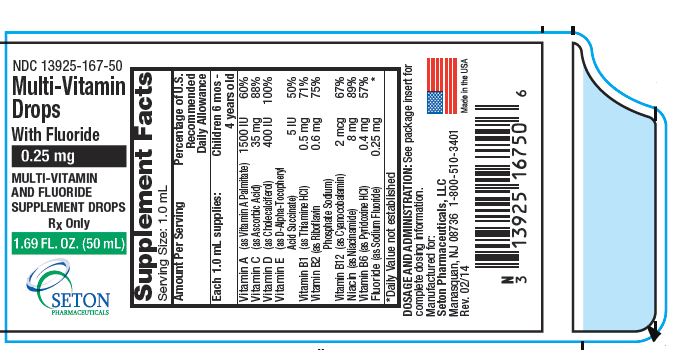
0.25 mg
|
||
| Serving Size: 1.0 mL | ||
| Amount Per Serving | Percentage of U.S. Recommended Daily Allowance | |
|---|---|---|
| Each 1.0 mL supplies: | Children 6 mos - 4 years old | |
| Vitamin A (as Vitamin A Palmitate) | 1500 IU | 60% |
| Vitamin C (as Ascorbic Acid) | 35 mg | 88% |
| Vitamin D (as Cholecalciferol) | 400 IU | 100% |
| Vitamin E (as d-alpha-tocopheryl acid succinate) | 5 IU | 50% |
| Vitamin B1 (as Thiamine HCI) | 0.5 mg | 71% |
| Vitamin B2 (as Riboflavin Phosphate Sodium) | 0.6 mg | 75% |
| Vitamin B12 (as Cyanocobalamin) | 2 mcg | 67% |
| Niacin (as Niacinamide) | 8 mg | 89% |
| Vitamin B6 (as Pyridoxine HCl) | 0.4 mg | 57% |
| Fluoride (as Sodium Fluoride) | 0.25 mg | * |
Active ingredient for caries prophylaxis: Fluoride as sodium fluoride. This product does not contain Folic Acid.
Other Ingredients: Caramel color, cherry flavor, ferrous sulfate, oil of orange, glycerin, methylparaben, polysorbate 80, purified water, sodium benzoate and sodium hydroxide.
| Age | <0.3 ppm | 0.3-0.6 ppm | >0.6 ppm |
|---|---|---|---|
| Birth - 6 months | None | None | None |
| 6 mos - 3 years | 0.25 mg (1 mL) / day† | None | None |
| 3 - 6 years | 0.50 mg (2 mL) / day | 0.25 mg (1 mL) / day | None |
HOW SUPPLIED
Multi-Vitamin and Fluoride 0.25 mg drops is available in 50 mL bottles with accompanying calibrated dropper.

RECOMMENDED STORAGE
Store at controlled room temperature 15°-25°C (between 59°F and 77°F). Excursions Permitted. After opening store away from direct light. Close tightly after each use. Occasional deepening of color has no significant effect on vitamin potency. REFRIGERATION IS NOT REQUIRED. SHAKE WELL.
Manufactured for:
Seton Pharmaceuticals, LLC
Manasquan, NJ 08736
1-800-510-3401
Rev. 02/14
Made in the USA
| MULTI VITAMIN DROPS WITH FLUORIDE
vitamin a palmitate, ascorbic acid, cholecalciferol, alpha-tocopherol succinate, d-thiamine hydrochloride, riboflavin 5-phosphate sodium, cyanocobalamin, niacinamide, pyridoxine hydrochloride and sodium fluoride solution/ drops |
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
| Labeler - Seton Pharmaceuticals (828898002) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Sonar Products | 104283945 | manufacture(13925-167) | |