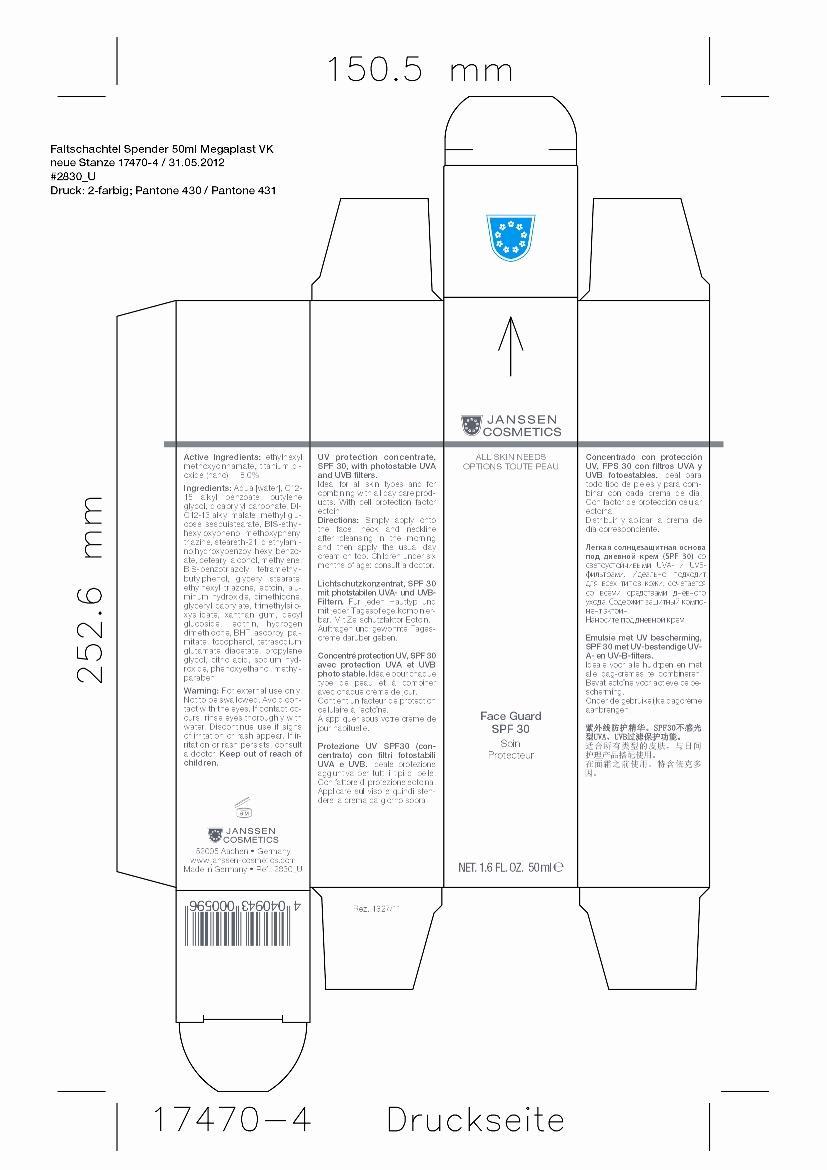
Label: FACE GUARD 30 SPF- octinoxate and titanium dioxide cream
-
Contains inactivated NDC Code(s)
NDC Code(s): 24653-830-01, 24653-830-02 - Packager: Janssen Cosmetics GmbH
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated October 9, 2012
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
-
Inactive Ingredients
Aqua [water], C12-15 alkyl benzoate, butylenes glycol, dicaprylyl carbonate, DI-C12-13 alkyl malate, methyl glucose sesquistearate, BIS-ethylhexyloxyphenol methoxyphenyl triazine, steareth-21, diethylamino hydroxybenzoyl hexyl benzoate, cetearyl alcohol, methylene,BIS-benzotriazolyl tetramethylbutylphenol, glyceryl stearate,ethylhexyl triazone, ectoin, aluminum hydroxide, dimethicone, glyceryl caprylate, trimethylsiloxysilicate, xanthan gum, decyl glucoside, lecithin, hydrogen dimethicone, BHT, ascorbyl palmitate, tocopherol, tetrasodium glutamate diacetate, propylene glycol, citric acid, sodium hydroxide, phenoxyethanol, methylparaben
- Ask a Doctor
- Directions
- WARNINGS
- Front Panel of Box
-
INGREDIENTS AND APPEARANCE
FACE GUARD 30 SPF
octinoxate and titanium dioxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:24653-830 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 3.5 g in 50 mL TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE .5 g in 50 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) C12-15 ALKYL BENZOATE (UNII: A9EJ3J61HQ) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) DICAPRYLYL CARBONATE (UNII: 609A3V1SUA) DI-C12-15 ALKYL FUMARATE (UNII: A1CB3Z898P) GLUCAMETACIN (UNII: N1EXE5EHAN) BEMOTRIZINOL (UNII: PWZ1720CBH) STEARETH-21 (UNII: 53J3F32P58) DIETHYLAMINO HYDROXYBENZOYL HEXYL BENZOATE (UNII: ANQ870JD20) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) BISOCTRIZOLE (UNII: 8NT850T0YS) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) ETHYLHEXYL TRIAZONE (UNII: XQN8R9SAK4) ECTOINE (UNII: 7GXZ3858RY) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) DIMETHICONE (UNII: 92RU3N3Y1O) GLYCERYL CAPRYLATE (UNII: TM2TZD4G4A) TRIMETHYLSILOXYSILICATE (M/Q 0.6-0.8) (UNII: 5041RX63GN) XANTHAN GUM (UNII: TTV12P4NEE) DECYL GLUCOSIDE (UNII: Z17H97EA6Y) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) ASCORBYL PALMITATE (UNII: QN83US2B0N) TOCOPHEROL (UNII: R0ZB2556P8) TETRASODIUM GLUTAMATE DIACETATE (UNII: 5EHL50I4MY) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) CITRIC ACID ACETATE (UNII: DSO12WL7AU) SODIUM HYDROXIDE (UNII: 55X04QC32I) PHENOXYETHANOL (UNII: HIE492ZZ3T) METHYLPARABEN (UNII: A2I8C7HI9T) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:24653-830-02 1 in 1 BOX 1 NDC:24653-830-01 50 mL in 1 BOTTLE, PUMP Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 06/01/2012 Labeler - Janssen Cosmetics GmbH (499187946) Registrant - Janssen Cosmetics GmbH (499187946) Establishment Name Address ID/FEI Business Operations Janssen Cosmetics GmbH 499187946 manufacture(24653-830)