MORPHINE SULFATE- morphine sulfate injection
Amphastar Pharmaceuticals, Inc.
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
MORPHINE SULFATE
INJECTION, USP CII
DESCRIPTION
Morphine Sulfate Injection USP is a sterile, nonpyrogenic solution of Morphine Sulfate USP in a citrate buffer. This product is to be administered by the intravenous route with the LifeCare PCA Infuser. It is not for intrathecal or epidural use.
Each mL contains morphine sulfate, pentahydrate 1 mg, sodium chloride 7.6 mg, with citric acid, anhydrous 0.4 mg and sodium citrate, dihydrate 0.2 mg added as buffers. Sodium metabisulfite 0.9 mg is added as antioxidant. May contain additional citric acid and/or sodium citrate for pH adjustment to USP limits of 2.5 to 6.5.
The solution contains no bacteriostat or antimicrobial agent and is intended only as a single-dose unit to provide multiple injections via a LifeCare® PCA Infuser. When the dosing requirement is completed, the unused portion should be discarded in an appropriate manner.
Morphine, the principal alkaloid of opium, is classified pharmacologically as a narcotic analgesic.
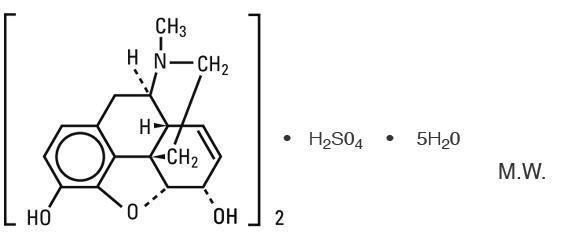
Morphine Sulfate USP (pentahydrate) is chemically designated 7, 8-didehydro-4, 5α-epoxy-17-methylmorphinan-3,6α-diol sulfate pentahydrate, a white crystalline powder, soluble in water.
The molecular formula is (C17H19NO3)2 • H2SO4 • 5H2O, and the structural formula is as follows:
Sodium Chloride USP is chemically designated NaCl, a white crystalline compound freely soluble in water.
CLINICAL PHARMACOLOGY
Morphine, an opium alkaloid, is a narcotic analgesic and provides analgesia at a dose that does not produce severe alterations in consciousness. Its principal therapeutic effect is relief of pain. Its exact mechanism and locus of action are not known, but are believed to relate to the existence of opiate receptors in the central nervous system. The drug affects both the initial perception of pain and the emotional response to it and although pain relief is not usually complete, the level of distress or suffering is markedly decreased. In addition to analgesia, narcotics produce drowsiness, changes in mood, and mental clouding; however, neither sensory modalities nor motor activity are blocked at therapeutic doses. There is no intrinsic limit to the analgesic effect, but high dosages can produce adverse effects such as respiratory depression, nausea and vomiting, cough reflex depression, miosis, mild vasodilation and an increase in tone of the gastrointestinal and genitourinary tracts. The use of Morphine Sulfate Injection USP in the LifeCare PCA Infuser should not result in true patient addiction.
Pain relief generally begins within several minutes after I.V. injection. Higher doses provide greater analgesic effect and longer duration of action but adverse effects limit the maximum tolerated dose.
Morphine is detoxified in the liver by conjugation and glucuronic acid. Small amounts of the free drug and larger amounts of conjugated morphine are found in the urine. These account for most of the administered drug and 90% of the total excretion occurs within the first 24 hours.
INDICATIONS AND USAGE
Morphine Sulfate Injection USP administered by slow intravenous injection is indicated for the relief of moderate to severe pain.
CONTRAINDICATIONS
Morphine is contraindicated in patients who are hypersensitive to the drug. Morphine is also contraindicated in patients who are receiving monoamine oxidase (MA0) inhibitors or who have received such agents within 14 days.
This product is contraindicated in patients known to be hypersensitive to bisulfites.
WARNINGS
Drug Dependence
Morphine sulfate can produce drug dependence and, therefore, has a potential for being abused. Tolerance, psychological dependence and physical dependence may develop upon repeated administration. Abrupt cessation after prolonged use may result in withdrawal symptoms and morphine sulfate should be WITHDRAWN GRADUALLY from any patient known to be taking excessive dosage over long periods of time (see DRUG ABUSE AND DEPENDENCE).
Infants born to mothers physically dependent on morphine sulfate may also be physically dependent and exhibit respiratory depression and withdrawal symptoms (see DRUG ABUSE AND DEPENDENCE).
Respiratory Depression
Dose related respiratory depression is produced by morphine acting directly on brain stem respiratory centers. Morphine also affects centers controlling respiratory rhythm and may produce irregular or periodic breathing. If significant respiratory depression occurs, it may be reversed by the use of naloxone hydrochloride (see OVERDOSAGE).
Head Injury and Increased Intracranial Pressure
Respiratory depressant effects produced by narcotics and their tendency to elevate cerebrospinal fluid pressure may be markedly exaggerated in the presence of head injury, other intracranial lesions, or a pre-existing increase in intracranial pressure. Furthermore, narcotics can produce adverse reactions which may obscure the clinical course of patients with head injuries. In such patients, morphine must be used with extreme caution and only if its use is deemed essential.
Acute Abdominal Conditions
Administration of narcotics may obscure the diagnosis or clinical course of patients with acute abdominal conditions.
Contains sodium metabisulfite, a sulfite that may cause allergic-type reactions including anaphylactic symptoms and life-threatening or less severe asthmatic episodes in certain susceptible people. The overall prevalence of sulfite sensitivity in the general population is unknown and probably low. Sulfite sensitivity is seen more frequently in asthmatic than in nonasthmatic people.
PRECAUTIONS
General
Morphine should be used with caution, and the initial dose reduced in certain patients, such as the elderly or debilitated and those with impaired hepatic or renal function, hypothyroidism, Addison's disease and prostatic hypertrophy or urethral stricture. Indiscriminate use of morphine in asthma and pulmonary emphysema may, due to its drying action upon the respiratory tract mucosa, precipitate severe respiratory insufficiency resulting from increased viscosity of bronchial secretions and suppression of the cough reflex. Morphine should be used with great caution in patients having an acute asthmatic attack, in those with chronic obstructive pulmonary disease or cor pulmonale, and in individuals with a substantially decreased respiratory reserve, pre-existing respiratory depression, hypoxia or hypercapnia. As with any narcotic analgesic agent, the usual precautions should be observed and the possibility of respiratory depression should be kept in mind.
Drug Interactions
Patients receiving other narcotic analgesics, general anesthetics, cimetidine, tranquilizers, sedative-hypnotics, MAO inhibitors, tricyclic antidepressants, or other CNS depressants (including alcohol) concomitantly with morphine may exhibit an additive CNS depression. Morphine analgesia may be decreased when given concomitantly with phenothiazines. When such combined therapy is contemplated, the dose of one or both agents should be appropriately adjusted.
Pregnancy Category C
Adequate animal studies on reproduction have not been performed to determine whether morphine sulfate (administered prior to term) affects fertility in males or females, or has teratogenic potential on the fetus. There are no well-controlled studies in pregnant women but marketing experience does not include any evidence of adverse effects on the fetus following routine (short-term) clinical use. Although there is no clearly defined risk, such experience cannot exclude the possibility of infrequent or subtle damage to the human fetus. Morphine sulfate should be used in pregnant women only when clearly needed (see Labor and Delivery and DRUG ABUSE AND DEPENDENCE).
Following maternal administration, this drug crosses the placenta and produces pharmacological effects in the fetus. Morphine sulfate administered a short time (i.e., up to 4 hours) prior to delivery to women with no history of chronic abuse or dependence has been associated with a delay in initial respiration and transient respiratory depression in the neonate. Respiratory depression may be produced in the neonate even in its absence in the mother, presumably because of an immature blood brain barrier in the neonate. The comparative fetal and neonatal respiratory depressant tendencies of equivalent analgesic doses of all drugs in this class have been determined.
Nonteratogenic Effects
Dependence has been reported in newborns whose mothers received opiates regularly during pregnancy. Withdrawal signs include irritability, excessive crying, tremors, hyperactive reflexes, fever, vomiting and diarrhea. Signs usually appear during the first few days after birth.
Labor and Delivery
As with all narcotics, administration of morphine to the mother during labor or shortly before delivery may result in some degree of respiratory depression in the newborn. Morphine prolongs labor. Naloxone may be used in the neonate to reverse morphine-induced respiratory depression.
Occasionally, morphine sulfate may prolong labor through actions which temporarily reduce the strength, duration and frequency of uterine contraction. However, this effect is not consistent and may be offset by an increased rate of cervical dilation which tends to shorten labor.
Morphine sulfate should be used with caution in women delivering premature infants since respiratory depression in the neonate may occur. Neonates whose mothers received morphine sulfate during labor should be observed closely for signs of respiratory depression. A specific narcotic antagonist, naloxone, is available for reversal of narcotic-induced respiratory depression in the neonate.
ADVERSE REACTIONS
Central Nervous System: Sedation, drowsiness, mental clouding, dizziness, lethargy, impairment of mental and physical performance, anxiety, convulsion, fear, miosis, dysphoria, psychic dependence, mood changes, and respiratory depression.
Gastrointestinal System: Nausea, vomiting, increased pressure in the biliary tract, constipation.
Cardiovascular System: Orthostatic hypotension, fainting, tachycardia, circulatory depression, peripheral circulatory collapse, and cardiac arrest have occurred after rapid intravenous injection.
Genitourinary System: Ureteral spasm, spasm of vesical sphincters and urinary retention have been reported.
Other: Flushing, sweating, pruritus, allergic reactions, suppressed cough reflex.
DRUG ABUSE AND DEPENDENCE
Morphine is a Schedule II controlled substance.
Psychic dependence, physical dependence and tolerance may develop upon repeated administration of narcotics; therefore, morphine should be prescribed and administered with caution. However, psychic dependence is unlikely to develop when morphine is used for a short time for the relief of pain. Physical dependence assumes clinically significant proportions only after several weeks of continued narcotic use, although some mild degree of physical dependence may develop after a few days of narcotic therapy. Tolerance is manifested initially by a shortened duration of analgesic effect, and subsequently by decreases in the intensity of analgesia. The rate of development of tolerance varies among patients. Withdrawal symptoms in patients dependent on morphine include yawning, sweating, lacrimation, rhinorrhea, restlessness, sleeplessness, dilated pupils, gooseflesh, irritability, tremor, nausea, vomiting and diarrhea. Treatment of the abstinence syndrome is primarily symptomatic and supportive, including maintenance of proper fluid and electrolyte balance.
OVERDOSAGE
Symptoms
Overdosage with morphine is characterized by respiratory depression (a decrease in respiratory rate and/or tidal volume, Cheyne-Stokes respiration, cyanosis), pinpoint pupils, extreme somnolence progressing to stupor or coma, skeletal muscle flaccidity, cold and clammy skin, and sometimes bradycardia and hypotension. In severe overdosage, particularly by the intravenous route, apnea, circulatory collapse, cardiac arrest and death may occur.
Treatment
Primary attention should be given to the re-establishment of adequate respiratory exchange through provision of a patent airway and institution of assisted or controlled ventilation. Naloxone hydrochloride is a specific and effective antagonist for respiratory depression which may result from overdosage or sensitivity to narcotics. The usual initial adult dose is 0.4 mg naloxone administered I.V. If the desired degree of counteraction and improvement in respiratory function is not obtained immediately following I.V. administration, it may be repeated intravenously at 2 to 3 minute intervals. Failure to obtain significant improvement after 2 or 3 doses suggests that the condition may be due partly or completely to other disease processes or nonopioid drugs. The usual initial pediatric dose is 0.01 mg/kg body weight given I.V., I.M. or S.C. This dose may be repeated in accordance with the adult administration guideline. If necessary, naloxone can be diluted with Sterile Water for Injection USP. Oxygen, intravenous fluids, vasopressors and other supportive measures should be employed as indicated. An antagonist should not be administered in the absence of clinically significant respiratory or cardiovascular depression. Morphine is not dialyzable.
Toxic dose of morphine in humans by parenteral routes: A dose in excess of 30 mg rapidly administered is likely to induce significant toxic effects in the nonaddicted adult who is not in pain.
Note: In an individual physically dependent on narcotics, the administration of the usual dose of a narcotic antagonist will precipitate an acute withdrawal dependence. The use of narcotic antagonists in such individuals should be avoided if possible. If a narcotic antagonist must be used to treat serious respiratory depression in the physically dependent patient, the antagonist should be administered with extreme care and only one-tenth to one-fifth the usual dose administered.
DOSAGE AND ADMINISTRATION
PHYSICIANS SHOULD COMPLETELY FAMILIARIZE THEMSELVES WITH THE LIFECARE PCA INFUSER BEFORE PRESCRIBING MORPHINE SULFATE INJECTION USP.
When administered intravenously, morphine sulfate should be given very slowly. Rapid intravenous injection increases the incidence of adverse reactions as described above. This drug should be administered intravenously only if a narcotic antagonist (i.e., naloxone) is available. When morphine sulfate is given parenterally, especially intravenously, the patient should be nonambulatory.
For use in a LifeCare PCA Infuser, dosage should be adjusted according to the severity of the pain and the response of the patient. There can be considerable variability in both the dosage requirement and patient response.
Incompatibility
Morphine Sulfate Injection USP, is incompatible with admixtures of soluble barbiturates, chlorothiazide, aminophylline, heparin, meperidine, methicillin, phenytoin, sodium bicarbonate, iodide, sulfadiazine and sulfisoxazole.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration. Do not use unless solution is clear and package is undamaged. Morphine products may discolor over a period of time. No loss of analgesic potency and no increase in toxicity has ever been demonstrated for such discolored solutions. Do not use the injection if it is darker than pale yellow or discolored in any other way, or if it contains a precipitate.
HOW SUPPLIED
Morphine Sulfate Injection USP, 30 mg/30 mL (1mg/mL)
| Stock No. 1931 | 1 mg/mL | PCA | 30 mL | NDC 0548-1931-00 |
STOCK NO. 1931 is supplied in unit-use packages containing one single dose vial and vial injector. FOR USE WITH COMPATIBLE ABBOTT PCA SET WITH INTEGRAL ANTI-SIPHON VALVE AND LIFECARE® PCA INFUSER.
Ten unit-use packages per carton..
These products are for use with the stated compatible infusion devices only.
Assembly Instructions
Use aseptic technique, do not assemble until ready to use. Remove caps from vial and vial injector. Thread vial into injector and rotate clockwise about 3 half turns or until vial is pierced by metal cannula. DO NOT PUSH VIAL INTO INJECTOR; THIS MAY CAUSE MISALIGNMENT. Remove cover from male luer. Attach to appropriate I.V. set. Refer to set Directions for Use. To load vial injector assembly into infusion device, refer to operating manual of infusion device.
*LifeCare® is a registered trademark of Abbott Laboratories, North Chicago, IL 60064, U.S.A.
Rx Only
Rev. 1-08
INTERNATIONAL MEDICATION SYSTEMS, LIMITED
So. El Monte, CA 91733, U.S.A.
An Amphastar Pharmaceuticals Company
6919310C
PRINCIPAL DISPLAY PANEL - 1 mg Carton
CAUTION: TO PREVENT OVERDOSE,
VIAL AND INJECTOR MUST BE
SECURELY LOCKED INTO VIAL
HOLDER AND INJECTOR HOLDER
OF INFUSER.
NDC 0548-1931-00
STOCK NO. 1931
CII
MORPHINE SULFATE
INJ., USP, (1 mg/mL)
30 mg
per
30 mL
FOR SLOW INTRAVENOUS USE / NARCOTIC ANALGESIC
NOT FOR INTRATHECAL OR EPIDURAL USE
FOR USE ONLY WITH COMPATIBLE ABBOTT PCA SET WITH
INTEGRAL ANTI-SIPHON VALVE AND LIFECARE® PCA INFUSER

| MORPHINE SULFATE
morphine sulfate injection |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Amphastar Pharmaceuticals, Inc. (024736733) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Amphastar Pharmaceuticals, Inc. | 024736733 | analysis(0548-1931) , manufacture(0548-1931) , label(0548-1931) | |