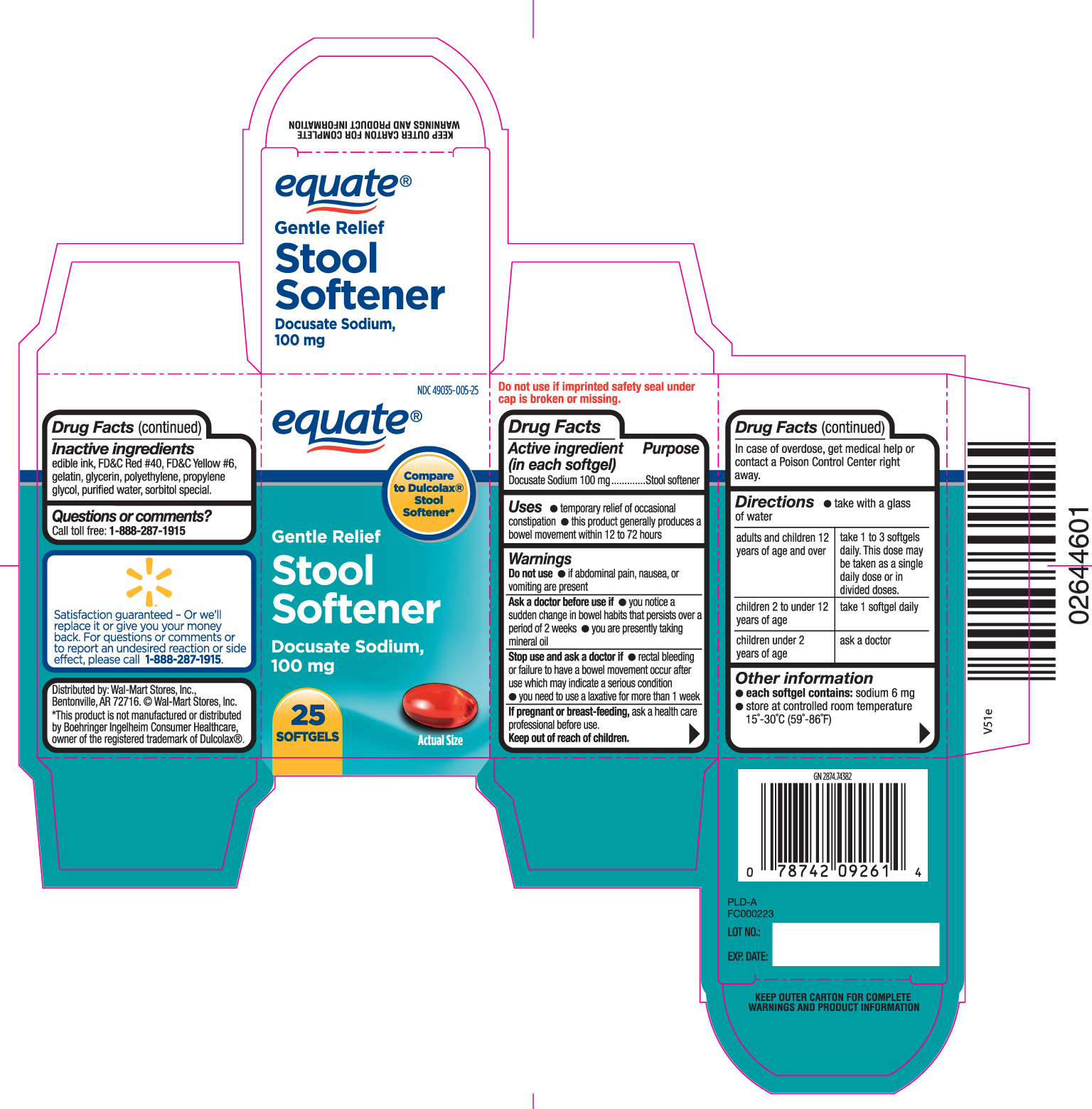
STOOL SOFTENER GENTLE RELIEF- docusate sodium capsule
EQUATE (Wal-Mart Stores, Inc.) (see also WAL-MART INC)
----------
Drug Facts
Uses
- temporary relief of occasional constipation
- this product generally produces a bowel movement within 12 to 72 hours.
Warnings - Do not use
- if abdominal pain, nausea or vomiting are present
Ask a doctor before use if
- you notice a sudden change in bowel habits that persists over a period of 2 weeks
- you are presently taking mineral oil
Directions
- take with a glass of water
| adults and children 12 years of age and over | take 1 to 3 softgels daily. This dose may be taken as a single daily dose or in divided doses. |
| children 2 to under 12 years of age | take 1 softgel daily |
| children under 2 years of age | ask a doctor |
Other information
- each softgel contains: sodium 6 mg
- store at controlled room temperature 15o - 30o C (59o- 86o F)
Inactive Ingredients
edible ink, FD&C Red #40, FD&C Yellow #6, gelatin, glycerin, polyethylene glycol, propylene glycol, purified water, sorbitol special.
Principal Display Panel
Compare to Dulcolax® Stool Softener*
Gentle Relief
stool softener
Docusate sodium 100 mg
DO NOT USE IF IMPRINTED SAFETY SEAL UNDER CAP IS BROKEN OR MISSING
KEEP OUTER CARTON FOR COMPLETE WARNINGS AND PRODUCT INFORMATION
Distributed by: Wal-mart stores, Inc., Bentonville, AR 72716
©Wal-mart stores, Inc.
*This product is not manufactured or distributed by Boehringer Ingelheim Consumer Healthcare owner of the registered trademark of Dulcolax®
| STOOL SOFTENER
GENTLE RELIEF
docusate sodium capsule |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - EQUATE (Wal-Mart Stores, Inc.) (see also WAL-MART INC) (051957769) |
| Registrant - P & L Development, LLC (079765031) |
Revised: 3/2024
Document Id: 9109beed-78c7-4de5-a87e-48f036427516
Set id: 2ec1e621-0f2d-49f9-952e-2a7ba8c62b22
Version: 2
Effective Time: 20240320
EQUATE (Wal-Mart Stores, Inc.) (see also WAL-MART INC)