AMMENS MEDICATED- zinc oxide powder
Botanicals Internacional S.A. de C.V.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
AMMENS MEDICATED POWDER - ORIGINAL MEDICATED FORMULA
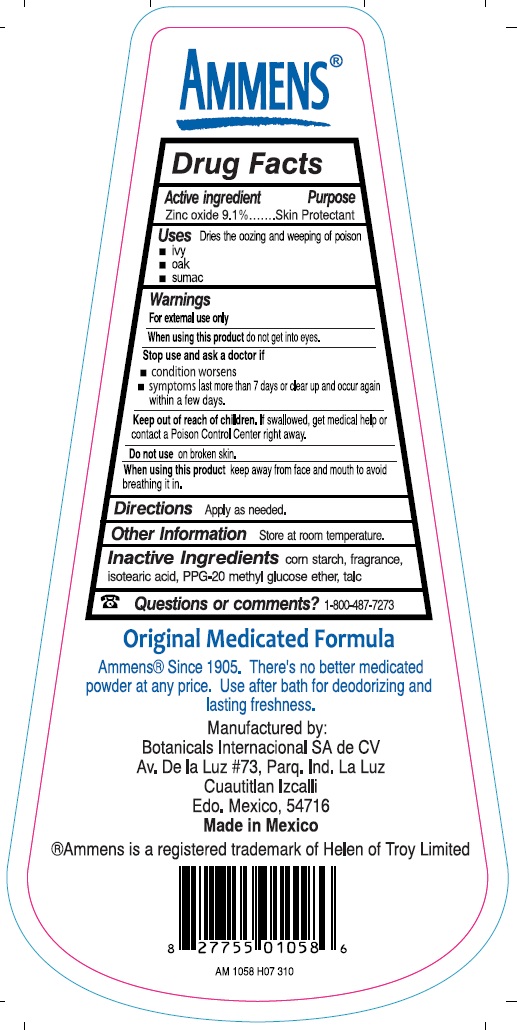
Stop use and ask a doctor if
condition worsens
symptoms last more than 7 days or clear up and occur again within a few days.
Keep out of reach of children. if swallowed, get medical help or contact a Poison Control Center right away.
Original Medicated Formula
Ammens(r) Since 1905. There's no better medicated powder at any price. Use after bath for deodorizing and lasting freshness.
Manufactured by:
Botanicals Internacional SA de CV
Av De la Luz #73 Parq. Ind. La Luz
Cuautitlan Izcalli
Edo. Mexico, 54716
Made in Mexico
Ammens is a registered trademark of Helen of Troy Lmited
| AMMENS MEDICATED
zinc oxide powder |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - Botanicals Internacional S.A. de C.V. (823968961) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Botanicals Internacional S.A. de C.V. | 823968961 | manufacture(51384-100) | |