Label: TELMISARTAN tablet
-
NDC Code(s):
65841-804-01,
65841-804-05,
65841-804-06,
65841-804-10, view more65841-804-16, 65841-804-30, 65841-804-78, 65841-805-01, 65841-805-05, 65841-805-06, 65841-805-10, 65841-805-16, 65841-805-30, 65841-805-78, 65841-806-01, 65841-806-05, 65841-806-06, 65841-806-10, 65841-806-16, 65841-806-30, 65841-806-78
- Packager: Zydus Lifesciences Limited
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated October 31, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
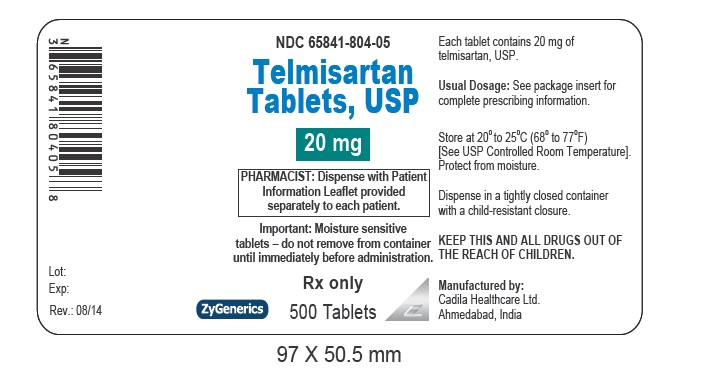
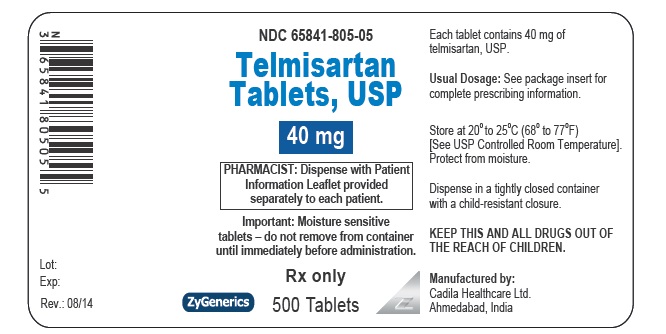
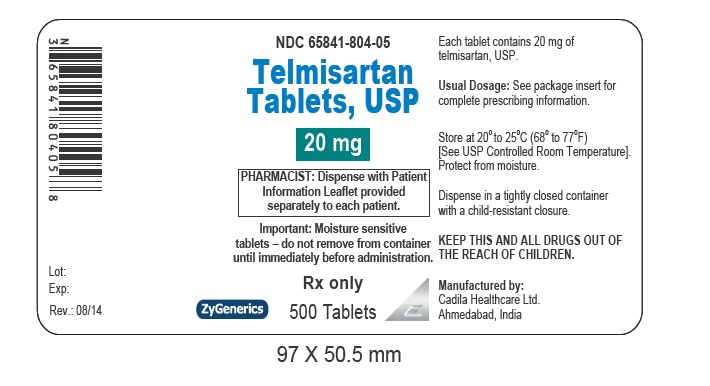
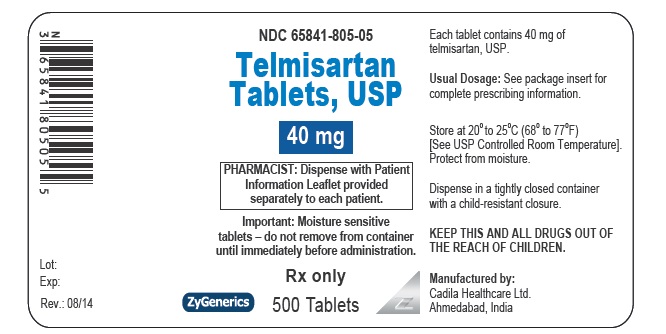
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
TELMISARTAN
telmisartan tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:65841-804 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TELMISARTAN (UNII: U5SYW473RQ) (TELMISARTAN - UNII:U5SYW473RQ) TELMISARTAN 20 mg Inactive Ingredients Ingredient Name Strength FERRIC OXIDE RED (UNII: 1K09F3G675) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) MEGLUMINE (UNII: 6HG8UB2MUY) POVIDONE (UNII: FZ989GH94E) SODIUM HYDROXIDE (UNII: 55X04QC32I) SODIUM STEARYL FUMARATE (UNII: 7CV7WJK4UI) SORBITOL (UNII: 506T60A25R) Product Characteristics Color BROWN (MOTTLED LIGHT BROWN TO MOTTLED BROWN) Score no score Shape ROUND (ROUND) Size 7mm Flavor Imprint Code 471 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:65841-804-06 30 in 1 BOTTLE; Type 0: Not a Combination Product 08/27/2014 2 NDC:65841-804-16 90 in 1 BOTTLE; Type 0: Not a Combination Product 08/27/2014 3 NDC:65841-804-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 08/27/2014 4 NDC:65841-804-05 500 in 1 BOTTLE; Type 0: Not a Combination Product 08/27/2014 5 NDC:65841-804-10 1000 in 1 BOTTLE; Type 0: Not a Combination Product 08/27/2014 6 NDC:65841-804-78 30 in 1 CARTON 08/27/2014 6 NDC:65841-804-30 1 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA203325 08/27/2014 TELMISARTAN
telmisartan tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:65841-805 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TELMISARTAN (UNII: U5SYW473RQ) (TELMISARTAN - UNII:U5SYW473RQ) TELMISARTAN 40 mg Inactive Ingredients Ingredient Name Strength SODIUM HYDROXIDE (UNII: 55X04QC32I) MEGLUMINE (UNII: 6HG8UB2MUY) POVIDONE (UNII: FZ989GH94E) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) FERRIC OXIDE RED (UNII: 1K09F3G675) SORBITOL (UNII: 506T60A25R) SODIUM STEARYL FUMARATE (UNII: 7CV7WJK4UI) MAGNESIUM STEARATE (UNII: 70097M6I30) Product Characteristics Color BROWN (MOTTLED LIGHT BROWN TO MOTTLED BROWN) Score no score Shape OVAL (OBLONG) Size 12mm Flavor Imprint Code 472 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:65841-805-06 30 in 1 BOTTLE; Type 0: Not a Combination Product 08/27/2014 2 NDC:65841-805-16 90 in 1 BOTTLE; Type 0: Not a Combination Product 08/27/2014 3 NDC:65841-805-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 08/27/2014 4 NDC:65841-805-05 500 in 1 BOTTLE; Type 0: Not a Combination Product 08/27/2014 5 NDC:65841-805-10 1000 in 1 BOTTLE; Type 0: Not a Combination Product 08/27/2014 6 NDC:65841-805-78 30 in 1 CARTON 08/27/2014 6 NDC:65841-805-30 1 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA203325 08/27/2014 TELMISARTAN
telmisartan tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:65841-806 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TELMISARTAN (UNII: U5SYW473RQ) (TELMISARTAN - UNII:U5SYW473RQ) TELMISARTAN 80 mg Inactive Ingredients Ingredient Name Strength SODIUM HYDROXIDE (UNII: 55X04QC32I) MEGLUMINE (UNII: 6HG8UB2MUY) POVIDONE (UNII: FZ989GH94E) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) FERRIC OXIDE RED (UNII: 1K09F3G675) SORBITOL (UNII: 506T60A25R) SODIUM STEARYL FUMARATE (UNII: 7CV7WJK4UI) MAGNESIUM STEARATE (UNII: 70097M6I30) Product Characteristics Color BROWN (MOTTLED LIGHT BROWN TO MOTTLED BROWN) Score no score Shape OVAL (OBLONG) Size 18mm Flavor Imprint Code 473 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:65841-806-06 30 in 1 BOTTLE; Type 0: Not a Combination Product 08/27/2014 2 NDC:65841-806-16 90 in 1 BOTTLE; Type 0: Not a Combination Product 08/27/2014 3 NDC:65841-806-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 08/27/2014 4 NDC:65841-806-05 500 in 1 BOTTLE; Type 0: Not a Combination Product 08/27/2014 5 NDC:65841-806-10 1000 in 1 BOTTLE; Type 0: Not a Combination Product 08/27/2014 6 NDC:65841-806-78 30 in 1 CARTON 08/27/2014 6 NDC:65841-806-30 1 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA203325 08/27/2014 Labeler - Zydus Lifesciences Limited (918596198) Registrant - Zydus Lifesciences Limited (918596198) Establishment Name Address ID/FEI Business Operations Zydus Lifesciences Limited 918596198 ANALYSIS(65841-804, 65841-805, 65841-806) , MANUFACTURE(65841-804, 65841-805, 65841-806)