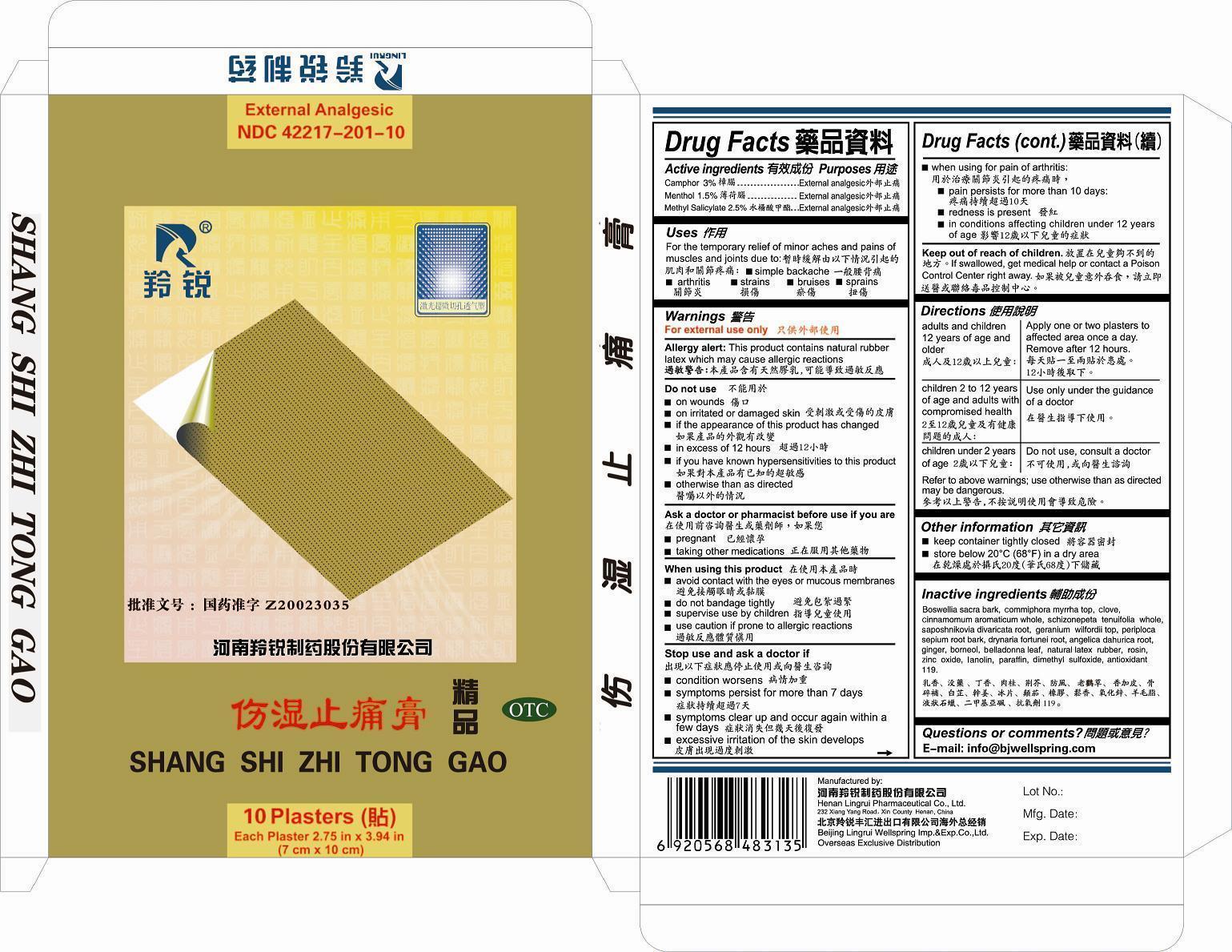
Label: SHANG SHI ZHI TONG GAO- camphor, menthol, methyl salicylate plaster
-
Contains inactivated NDC Code(s)
NDC Code(s): 42217-201-10 - Packager: Henan Lingrui Pharmaceutical Co.; Ltd
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated February 27, 2014
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENTS
- PURPOSE
- USES
- WARNINGS
- DO NOT USE
- Ask a Doctor or Pharmacist before use if you are
- When using this product
- Stop use and ask a doctor if
- Keep out of reach of children.
-
Adults and children 12 years of age and older: Apply one or two plasters to affected area once a day. Remove after 12 hours.DIRECTIONBRefer to the above warnings: use otherwise than as directed may be dangerous.
Adults and children 12 years of age and older: Apply one or two plasters to affected area once a day. Remove after 12 hours.
Children 2 to 12 years of age and adults with compromised health: Use only under the guidance of a doctor.
Children under 2 years of age: Do not use, consult a doctor.
Refer to the above warnings: use otherwise than as directed may be dangerous. - OTHER INFORMATION
-
INACTIVE INGREDIENTS
BOSWELLIA SACRA BARK, COMMIPHORA MYRRHA TOP, CLOVE, CINNAMOMUM AROMATICUM WHOLE, SCHIZONEPETA TENUIFOLIA WHOLE, SAPOSHNIKOVIA DIVARICATA ROOT, GERANIUM WILFORDII TOP, PERIPLOCA SEPIUM ROOT BARK, DRYNARIA FORTUNEI ROOT, ANGELICA DAHURICA ROOT, GINGER, BORNEOL, BELLADONNA LEAF, NATURAL LATEX RUBBER, ROSIN, ZINC OXIDE, LANOLIN, PARAFFIN, DIMETHYL SULFOXIDE, ANTIOXIDANT 119
- Questions or Commnets?
- DRUG FACTS
-
INGREDIENTS AND APPEARANCE
SHANG SHI ZHI TONG GAO
camphor, menthol, methyl salicylate plasterProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:42217-201 Route of Administration TRANSDERMAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CAMPHOR (NATURAL) (UNII: N20HL7Q941) (CAMPHOR (NATURAL) - UNII:N20HL7Q941) CAMPHOR (NATURAL) 3.0 g in 100 g MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 1.5 g in 100 g METHYL SALICYLATE (UNII: LAV5U5022Y) (SALICYLIC ACID - UNII:O414PZ4LPZ) METHYL SALICYLATE 2.5 g in 100 g Inactive Ingredients Ingredient Name Strength BOSWELLIA SACRA BARK (UNII: 1UK28DX728) COMMIPHORA MYRRHA TOP (UNII: N534J96ODY) CLOVE (UNII: K48IKT5321) CINNAMOMUM AROMATICUM WHOLE (UNII: 9BPF21T8ZR) SCHIZONEPETA TENUIFOLIA WHOLE (UNII: C1107616TR) SAPOSHNIKOVIA DIVARICATA ROOT (UNII: 8H84LFK2QD) GERANIUM WILFORDII TOP (UNII: 771B1S9W3R) PERIPLOCA SEPIUM ROOT BARK (UNII: 638OW484M3) DRYNARIA FORTUNEI ROOT (UNII: 731W842X8Q) ANGELICA DAHURICA ROOT (UNII: 1V63N2S972) GINGER (UNII: C5529G5JPQ) BORNEOL (UNII: M89NIB437X) BELLADONNA LEAF (UNII: 6GZW20TIOI) NATURAL LATEX RUBBER (UNII: 2LQ0UUW8IN) ROSIN (UNII: 88S87KL877) ZINC OXIDE (UNII: SOI2LOH54Z) LANOLIN (UNII: 7EV65EAW6H) PARAFFIN (UNII: I9O0E3H2ZE) DIMETHYL SULFOXIDE (UNII: YOW8V9698H) ANTIOXIDANT 119 (UNII: UBL01213LI) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:42217-201-10 10 in 1 BOX 1 1 g in 1 PATCH Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 06/02/2010 Labeler - Henan Lingrui Pharmaceutical Co.; Ltd (530021062) Registrant - Henan Lingrui Pharmaceutical Co.; Ltd (530021062) Establishment Name Address ID/FEI Business Operations Henan Lingrui Pharmaceutical Co.; Ltd 530021062 manufacture(42217-201)