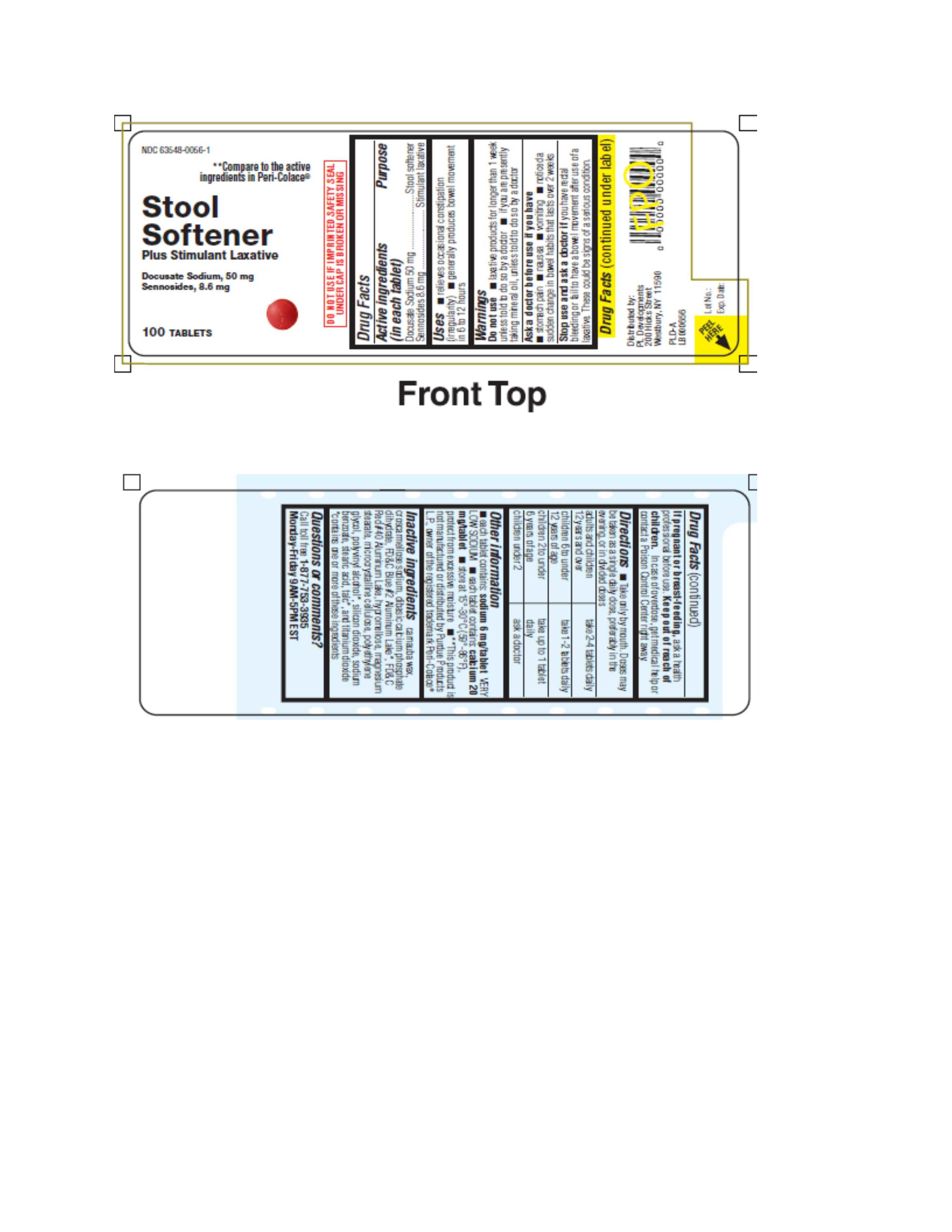
STOOL SOFTENER PLUS STIMULANT LAXATIVE- docusate sodium and sennosides tablet
PLD Acquisitions LLC DBA Avéma Pharma Solutions
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Stool Softener plus Stimulant Laxative
Uses
- relieves occasional constipation (irregularity)
- generally produces a bowel movement within 6 to 12hours.
Warnings
Do not use
- laxative products for longer than 1 week unless told to do so by a doctor.
- if you are presently taking mineral oil, unless told to do so by a doctor.
Ask a doctor before use if you have
- stomach pain
- nausea
- vomiting
- noticed a sudden change in bowel habits that lasts over 2 weeks.
Directions
Take only by mouth. Doses may be taken as a single daily does or in divided doses.
- adults and children 12 years and over: take 2-4 tablets daily
- children 6 to under 12 years of age: take 1-2 tablets daily
- children 2 to under 6 years of age: take up to 1 tablet daily
- children under 2: ask a doctor
Other information
- each tablet contains:sodium 6mg/tablet VERY LOW SODIUM
- each tablet contains: calcium 20mg/tablets
- store at 15 - 30 degrees C (59-86 degrees F), protect from excessive moisture
- **This product is not manufactured or distributed by Purdue Products, LP, owner of the registered trademark Peri Colace®.
Inactive Ingredients
carnauba wax, croscarmellose sodium, dibasic calcium phosphate dihydrate, FD&C Blue #2 Aluminum Lake*, FD&C Red #40 Aluminum Lake, hypromellose, magnesium stearate, microcrystalline cellulose, polyethylene glycol, polyvinyl alcohol*, silicon dioxide, sodium benzoate, stearic acid, talc*, and titanium dioxide
*contains one or more of these ingredients
| STOOL SOFTENER PLUS STIMULANT LAXATIVE
docusate sodium and sennosides tablet |
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
| Labeler - PLD Acquisitions LLC DBA Avéma Pharma Solutions (804087794) |