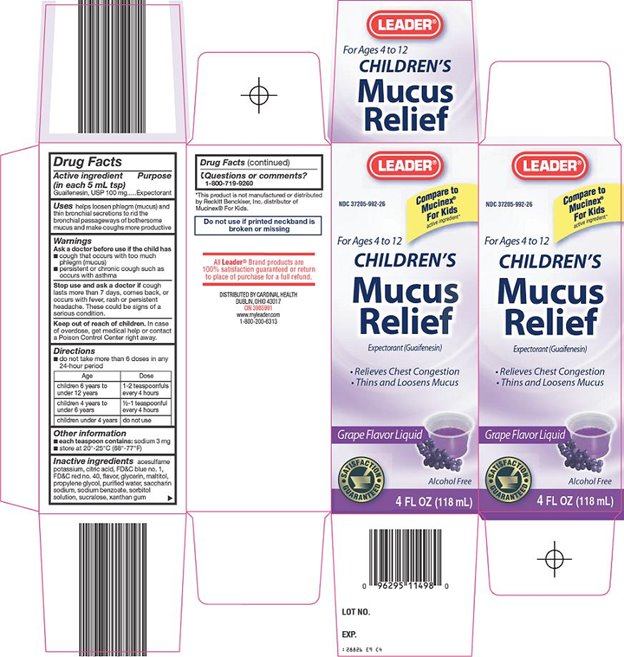
LEADER MUCUS RELIEF CHILDRENS- guaifenesin liquid
Cardinal Health
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Cardinal Health Children's Mucus Relief Drug Facts
Uses
helps loosen phlegm (mucus) and thin bronchial secretions to rid the bronchial passageways of bothersome mucus and make coughs more productive
Warnings
Ask a doctor before use if the child has
- •
- cough that occurs with too much phlegm (mucus)
- •
- persistent or chronic cough such as occurs with asthma
Directions
- •
- do not take more than 6 doses in any 24-hour period
|
Age |
Dose |
|
children 6 years to under 12 years |
1-2 teaspoonfuls every 4 hours |
|
children 4 years to under 6 years |
½ - 1 teaspoonful every 4 hours |
|
children under 4 years |
do not use |
| LEADER MUCUS RELIEF
CHILDRENS
guaifenesin liquid |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Cardinal Health (097537435) |
Revised: 11/2017
Document Id: b9aa31a6-d819-4cb5-b30b-51559b012a45
Set id: 2cf666d1-fd63-4a8d-a3eb-d0aa8765ff41
Version: 2
Effective Time: 20171113
Cardinal Health