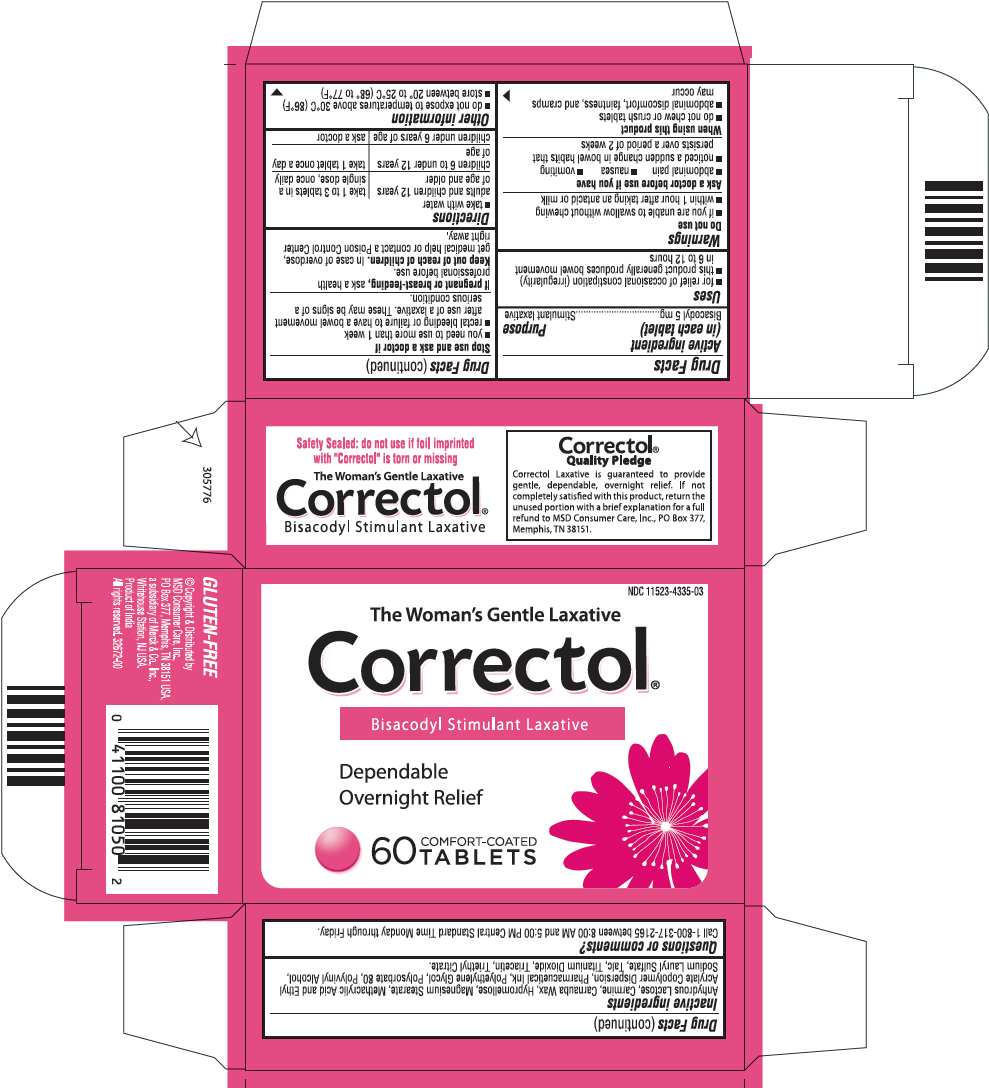
CORRECTOL- bisacodyl tablet, coated
Bayer HealthCare LLC.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Correctol ®
Uses
- for relief of occasional constipation (irregularity)
- this product generally produces bowel movement in 6 to 12
Warnings
Ask a doctor before use if you have
- abdominal pain
- nausea
- vomiting
- noticed a sudden change in bowel habits that persists over a period of 2 weeks
When using this product
- do not chew or crush tablets
- abdominal discomfort, faintness, and cramps may occur
Directions
- take with water
| adults and children 12 years of age and older | Take 1 to 3 tablets in a Single dose, once daily |
| children 6 to under 12 years of age | Take 1 tablet once a day |
| children under 6 years of age | ask a doctor |
Inactive ingredients
Anhydrous Lactose, Carmine, Carnauba wax, Hypromellose, Magnesium stearate, Methacrylic Acid and Ethyl Acrylate Copolymer Dispersion, Pharmaceutical Ink, Polyethylene Glycol, Polysorbate 80, Polyvinyl Alcohol, Sodium Lauryl Sulfate, Talc, Titanium Dioxide, Triacetin, Triethyl Citrate
Questions or comments?
Call 1-800-317-2165 between 8:00 AM and 5:00 PM Central Standard Time Monday through Friday.
| CORRECTOL
bisacodyl tablet, coated |
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Bayer HealthCare LLC. (112117283) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Rottendorf Pharma INC. (Germany) | 315974691 | manufacture(11523-4335) | |
Revised: 9/2017
Document Id: 599e653d-4e23-328d-e053-2991aa0aee17
Set id: 2c199518-ae77-40a6-82ce-3aa24a685d4a
Version: 2
Effective Time: 20170920
Bayer HealthCare LLC.