SOFT CARE ANTISEPTIC SKIN CLEANSER II- triclosan liquid
Diversey, Inc.
----------
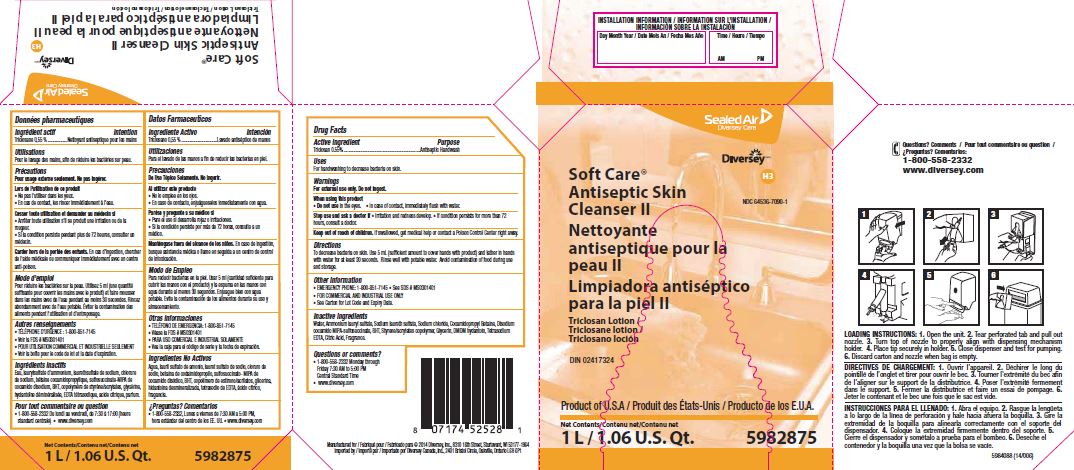
Drug Facts
Warnings
For external use only. Do not ingest.
Directions
To decrease bacteria on skin.
Use 5 mL (sufficient amount to cover hands with product) and lather in hands with water for at least 30 seconds.
Rinse well with potable water.
Avoid contamination of food during use and storage.
Inactive ingredients
Water, Ammonium lauryl sulfate, Sodium laureth sulfate, Sodium chloride, Cocamidopropyl betaine, Disodium cocamido MIPA-sulfosuccinate, BHT, Styrene/acrylates copolymer, Glycerin, DMDM hydantoin, Tetrasodium EDTA, Citric acid, Fragrance
Questions or comments?
1-800-558-2332 Monday through Friday 7:30 AM to 5:00 PM
Central Standard Time
www.diversey.com
| SOFT CARE ANTISEPTIC SKIN CLEANSER II
triclosan liquid |
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
| Labeler - Diversey, Inc. (018240817) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Kutol Products Company Inc | 004236139 | manufacture(64536-7090) | |
Revised: 11/2023
Document Id: 0a34706b-1e25-3e15-e063-6394a90a0327
Set id: 2b972659-629e-4805-9489-5a92d4a3e1cd
Version: 2
Effective Time: 20231115
Diversey, Inc.