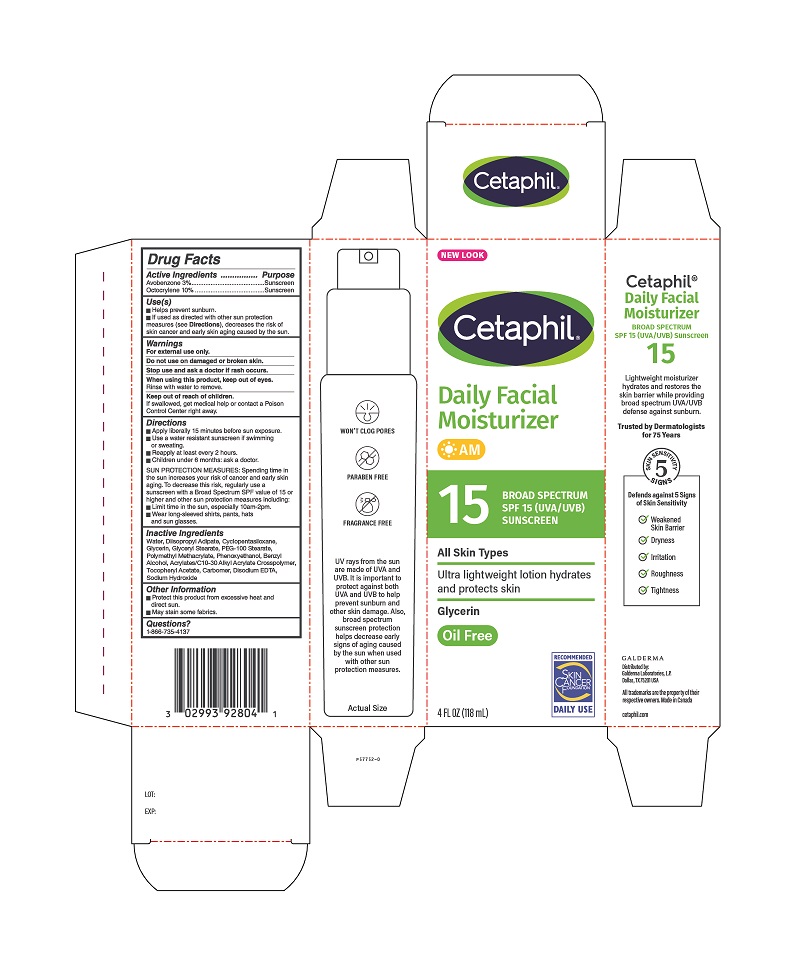
Label: CETAPHIL- octocrylene, avobenzone lotion
- NDC Code(s): 0299-3928-04
- Packager: Galderma Laboratories, L.P.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated April 16, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- USE(S)
- WARNINGS
- Keep out of reach of children.
-
DIRECTIONS
- Apply liberally 15 minutes before sun exposure.
- Use a water resistant sunscreen if swimming or sweating.
- Reapply at least every 2 hours.
- Children under 6 months: ask a doctor.
SUN PROTECTION MEASURES: Spending time in the sun increases your risk of cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including: - Limit time in the sun, especially 10am - 2pm.
- Wear long-sleeved shirts, pants, hats and sun glasses.
- INACTIVE INGREDIENTS:
- Other Information
- Questions?
-
PRINCIPAL DISPLAY PANEL - 4 FL OZ Carton
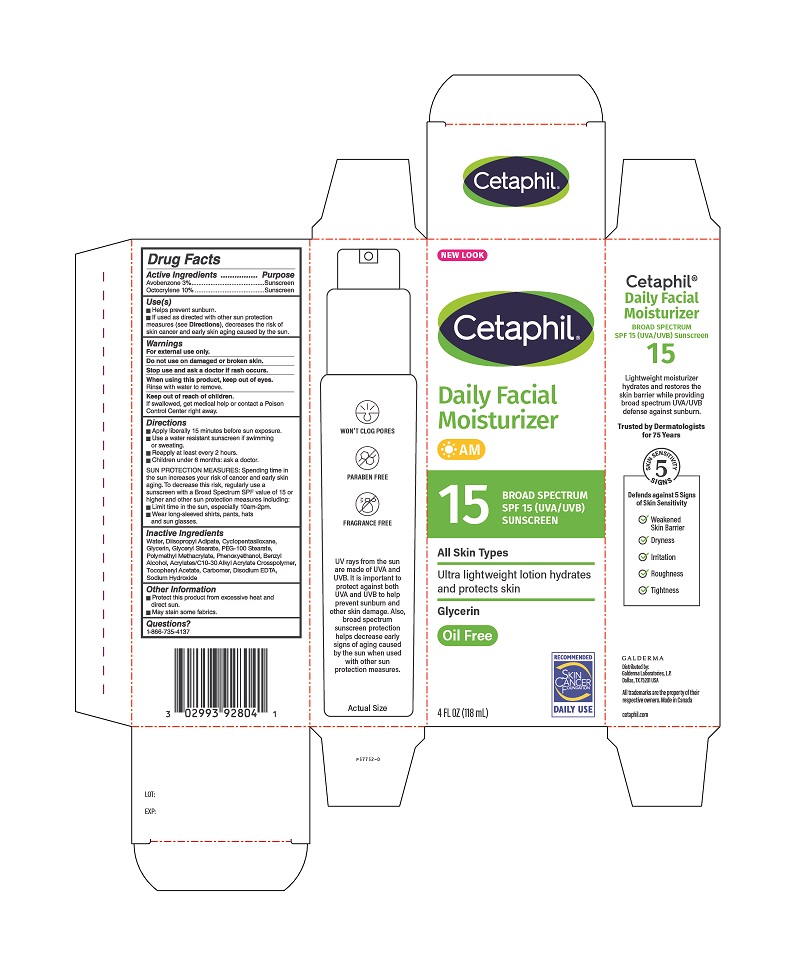
NEW LOOK
Cetaphil®Daily Facial
Moisturizer
AM15 BROAD SPECTRUM
SPF 15 (UVA/UVB)
SUNSCREEN
All Skin Types
Ultra lightweight lotion hydrates
and protects skinGlycerin
Oil-FreeRecommended
Skin Cancer Foundation
Daily Use4 FL OZ (118 mL)
Distributed by:
Galderma Laboratories, L.P.
Dallas, TX 75201 USAAll trademarks are the property of their respective owners.
Made in Canadacetaphil.com
P57752-0 -
INGREDIENTS AND APPEARANCE
CETAPHIL
octocrylene, avobenzone lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0299-3928 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 10 g in 100 mL AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 3 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) DIISOPROPYL ADIPATE (UNII: P7E6YFV72X) Cyclomethicone 5 (UNII: 0THT5PCI0R) GLYCERIN (UNII: PDC6A3C0OX) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) PEG-100 STEARATE (UNII: YD01N1999R) Poly(Methyl Methacrylate; 450000 Mw) (UNII: Z47NNT4J11) Phenoxyethanol (UNII: HIE492ZZ3T) Benzyl Alcohol (UNII: LKG8494WBH) Acrylates/C10-30 Alkyl Acrylate Crosspolymer (60000 Mpa.S) (UNII: 8Z5ZAL5H3V) .Alpha.-Tocopherol Acetate (UNII: 9E8X80D2L0) Carbomer Homopolymer, Unspecified Type (UNII: 0A5MM307FC) Edetate Disodium (UNII: 7FLD91C86K) Sodium Hydroxide (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0299-3928-04 1 in 1 CARTON 04/01/2000 1 118 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 04/01/2000 Labeler - Galderma Laboratories, L.P. (047350186) Establishment Name Address ID/FEI Business Operations G Production Inc. 251676961 manufacture(0299-3928)