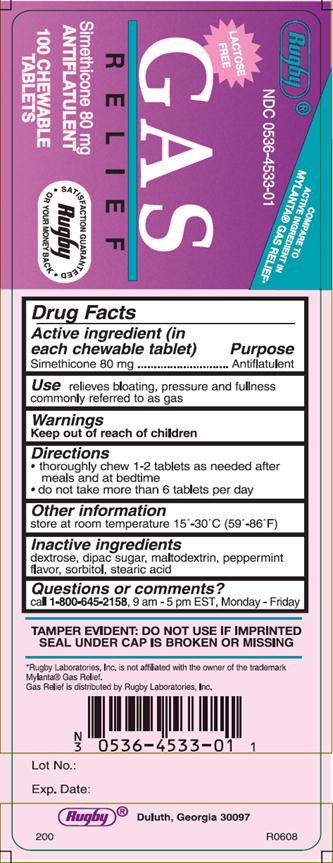
GAS RELIEF- simethicone tablet, chewable
Rugby Laboratories Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Gas Relief Antiflatulent Chewable Tablets
Directions
- •
- thoroughly chew 1-2 tablets as needed after meals and at bedtime
- •
- do not take more than 6 tablets per day
| GAS RELIEF
simethicone tablet, chewable |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Rugby Laboratories Inc. (079246066) |
Revised: 12/2019
Document Id: 81b0d040-e1df-4385-bb0e-be4eb5bee7c1
Set id: 28c3027d-85c0-4242-9b39-617c4d1ff44c
Version: 3
Effective Time: 20191219
Rugby Laboratories Inc.