Label: ATORVASTATIN CALCIUM tablet, film coated
-
Contains inactivated NDC Code(s)
NDC Code(s): 51655-880-30 - Packager: Northwind Pharmaceuticals
- This is a repackaged label.
- Source NDC Code(s): 0591-3777
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated April 28, 2014
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
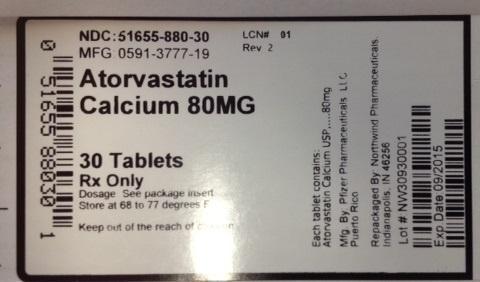
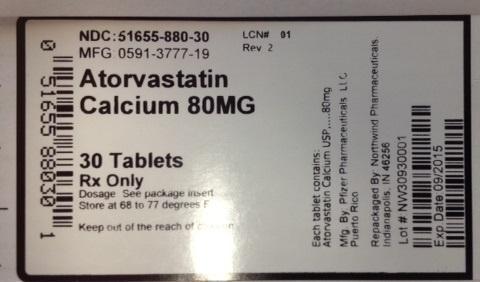
PRINCIPAL DISPLAY PANEL
NDC:51655-880-30
MFG: 0591-3777-19
Atorvastatin Calcium 80 mg
30 tablets
RX only
Dosage: See package insert
Store at 68 to 77 degrees F
Keep out of the reach of children.
Each tablet contains: Atorvastatin Calcium USP 80mg
Mfg By: Pfizer Pharmaceuticals, Puerto Rico
Repackaged by Northwind Pharmaceuticals Indianapolis, IN 46256
Lot # NW30930001
Exp Date: 09/2015
- WARNINGS AND PRECAUTIONS
-
INGREDIENTS AND APPEARANCE
ATORVASTATIN CALCIUM
atorvastatin calcium tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:51655-880(NDC:0591-3777) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ATORVASTATIN CALCIUM (UNII: 48A5M73Z4Q) (ATORVASTATIN - UNII:A0JWA85V8F) ATORVASTATIN 80 mg in 30 Product Characteristics Color white Score no score Shape capsule Size 19mm Flavor Imprint Code PD15880 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51655-880-30 30 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA020702 04/28/2014 Labeler - Northwind Pharmaceuticals (036986393) Registrant - Northwind Pharmaceuticals (036986393) Establishment Name Address ID/FEI Business Operations Northwind Pharmaceuticals 036986393 repack(51655-880)