ITCHY EYE- ketotifen solution
H E B
----------
HEB Itchy Eye Drops Drug Facts
Warnings
Do not use
- •
- if solution changes color or becomes cloudy
- •
- if you are sensitive to any ingredient in this product
- •
- to treat contact lens related irritation
When using this product
- •
- do not touch tip of container to any surface to avoid contamination
- •
- remove contact lenses before use
- •
- wait at least 10 minutes before reinserting contact lenses after use
- •
- replace cap after each use
Directions
- •
- Adults and children 3 years of age and older:
Put 1 drop in the affected eye(s) twice daily, every 8-12 hours, no more than twice per day.
- •
- Children under 3 years of age:
Consult a doctor.
Other information
- •
- only for use in the eye
- •
- store at 20-25°C (68-77°F) [see USP Controlled Room Temperature]
Inactive ingredients
benzalkonium chloride 0.01%, glycerin, purified water. May contain hydrochloric acid and/or sodium hydroxide (to adjust pH).
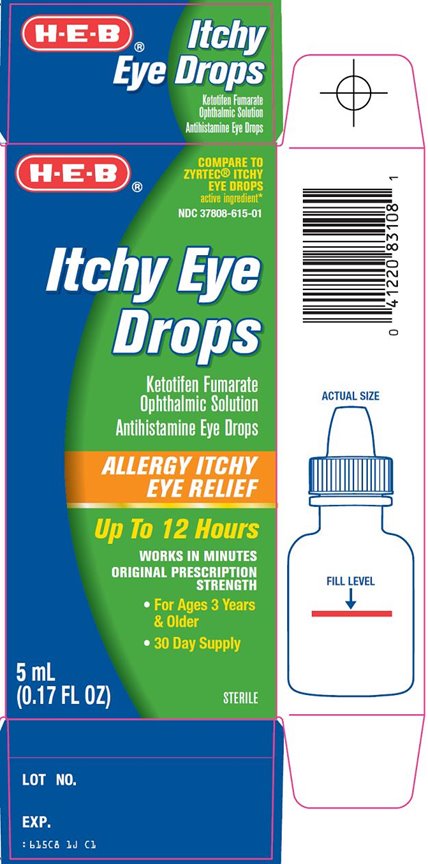
Package/Label Principal Display Panel
COMPARE TO ZYRTEC® ITCHY EYE DROPS active ingredient
Itchy Eye Drops
Ketotifen Fumarate Opthalmic Solution
Antihistamine Eye Drops
ALLERGY ITCHY EYE RELIEF
Up To 12 Hours
WORKS IN MINUTES
ORIGINAL PRESCRIPTION STRENGTH
For Ages 3 Years & Older
30 Day Supply
STERILE
Itchy Eye Drops Carton Image 2
| ITCHY EYE
ketotifen solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - H E B (007924756) |
Revised: 11/2017
Document Id: 30c0f2fc-b405-44c3-8b15-50e8b80f7af9
Set id: 27c98e1a-7600-4006-8041-b23d520841c4
Version: 3
Effective Time: 20171118
H E B