SLEEP AID NIGHTTIME- diphenhydramine hcl tablet
CVS Pharmacy
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
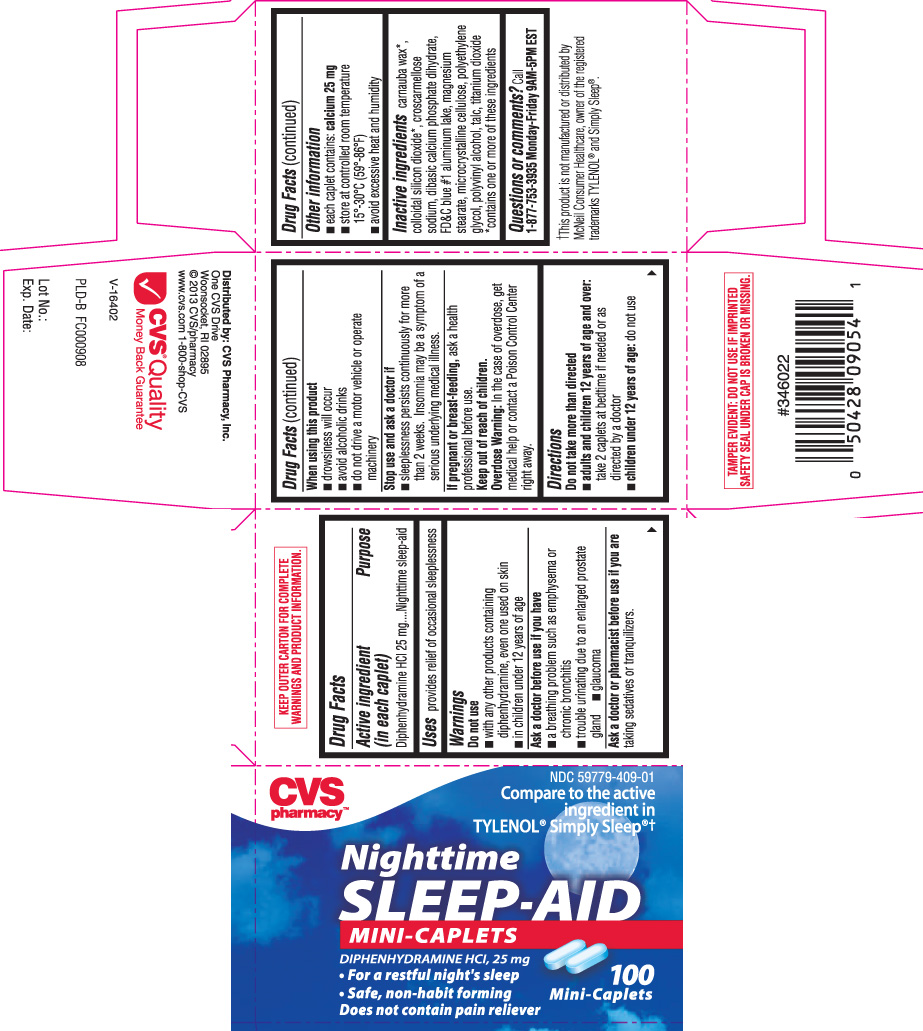
Drug Facts
Warnings
Do not use
- with any other products containing diphenhydramine, even one used on skin
- in children under 12 years of age
Ask a doctor before use if you have
- a breathing problem such as emphysema or chronic bronchitis
- trouble urinating due to an enlarged prostate gland
- glaucoma
When using this product
- drowsiness will occur
- avoid alcoholic drinks
- do not drive a motor vehicle or operate machinery
Directions
Do not take more than directed
- adults and children 12 years of age and over: take 2 caplets at bedtime if needed or as directed by a doctor
- children under 12 years of age: do not use
Other information
- each caplet contains: calcium 25 mg
- store at controlled room temperature 15°-30°C (59°-86°F)
- avoid excessive heat and humidity
Inactive ingredients
carnauba wax*, colloidal silicon dioxide*, croscarmellose sodium, dibasic calcium phosphate dihydrate, FD&C blue #1 aluminum lake, magnesium stearate, microcrystalline cellulose, polyethylene glycol, polyvinyl alcohol, talc, titanium dioxide
*contains one or more of these ingredients
Principal Display Panel
Compare to the active ingredient in TYLENOL® Simply Sleep®†
Nighttime SLEEP-AID
MINI-CAPLETS
DIPHENHYDRAMINE HCl, 25 mg
• For a restful night's sleep
• Safe, non-habit forming
Does not contain pain reliever
Mini-Caplets
†This product is not manufactured or distributed by McNeil Consumer Healthcare, owner of the registered trademarks TYLENOL® and Simply Sleep®.
Distributed by: CVS Pharmacy, Inc.
One CVS Drive
Woonsocket, RI 02895
KEEP OUTER CARTON FOR COMPLETE WARNINGS AND PRODUCT INFORMATION.
TAMPER EVIDENT: DO NOT USE IF IMPRINTED SAFETY SEAL UNDER CAP IS BROKEN OR MISSING.
| SLEEP AID
NIGHTTIME
diphenhydramine hcl tablet |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - CVS Pharmacy (062312574) |
| Registrant - P & L Development, LLC (800014821) |