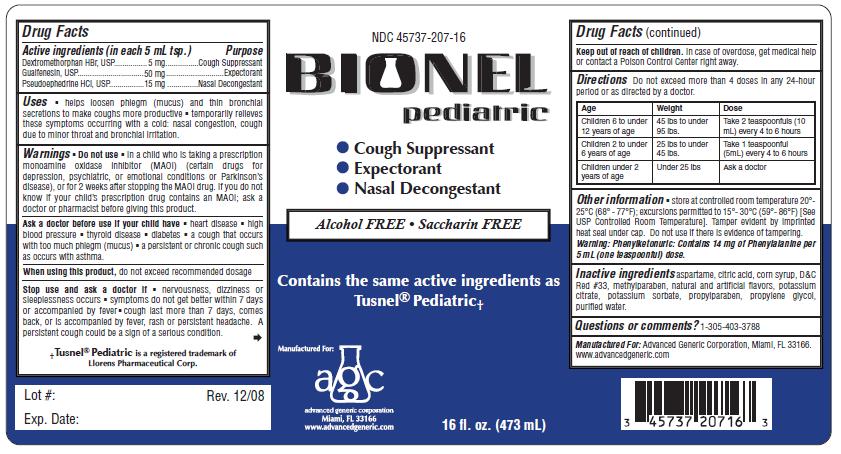
Label: BIONEL PEDIATRIC- dextromethorphan, guaifenesin, pseudoephedrine hydrochloride liquid
- NDC Code(s): 45737-207-16
- Packager: Advanced Generic Corporation
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated December 23, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
-
WARNINGS
Warnings
Do not use in a child who is taking a prescription
Monoamine Oxidase Inhibitor (MAOI) (certain drugs for depression,
psychiatric, or emotional conditions or Parkinson’s disease), or for
2 weeks after stopping the MAOI drug. If you do not know if you are
taking a prescription drug that contains a MAOI; ask a doctor or
pharmacist before taking this product. -
DO NOT USE
Ask a doctor before use if your child have
- heart disease
- high blood pressure
- thyroid disease
- diabetes
- difficulty in urination due to the enlargement of the prostate gland
- a cough that occurs with too much phlegm (mucus)
- a breathing problem or chronic cough that lasts or occurs with smoking, asthma, chronic bronchitis, or emphysema. A persistent cough may be a sign of a serious condition.
- you get nervous, dizzy or sleepless
- symptoms do not get better within 7 days or accompanied by fever
- cough lasts more than 7 days, comes back or is accompanied by fever, rash or persistent headache. These could be signs of a serious condition.
- KEEP OUT OF REACH OF CHILDREN
- PREGNANCY OR BREAST FEEDING
-
DOSAGE & ADMINISTRATION
Directions Do not exceed more than 4 doses in any 24-hour period or as directed by a doctor.
Children 6 to under 12 years of age; 45 lbs to under 95 lbs; Take 2 teaspoonfuls (10mL) every 4 to 6 hours
Children 2 to under 6 years of age; 25 lbs to under 45 lbs; Take 1 teaspoonful (5mL) every 4 to 6 hours
Children under 2 years of age; Under 25 lbs; Ask a doctor
-
INDICATIONS & USAGE
Other information
- store at controlled room temperature 20°- 25°C (68° - 77°F); excursion permitted to 15°- 30°C (59°- 86°F) [See USP Controlled Room Temperature]. Tamper evident by imprinted heat seal under cap. Do not use if there is evidence of tampering.
- store at controlled room temperature 20°- 25°C (68° - 77°F); excursion permitted to 15°- 30°C (59°- 86°F) [See USP Controlled Room Temperature]. Tamper evident by imprinted heat seal under cap. Do not use if there is evidence of tampering.
- INACTIVE INGREDIENT
- QUESTIONS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
BIONEL PEDIATRIC
dextromethorphan, guaifenesin, pseudoephedrine hydrochloride liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:45737-207 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Dextromethorphan Hydrobromide (UNII: 9D2RTI9KYH) (Dextromethorphan - UNII:7355X3ROTS) Dextromethorphan Hydrobromide 5 mg in 5 mL Guaifenesin (UNII: 495W7451VQ) (Guaifenesin - UNII:495W7451VQ) Guaifenesin 50 mg in 5 mL Pseudoephedrine Hydrochloride (UNII: 6V9V2RYJ8N) (Pseudoephedrine - UNII:7CUC9DDI9F) Pseudoephedrine Hydrochloride 15 mg in 5 mL Inactive Ingredients Ingredient Name Strength corn syrup (UNII: 9G5L16BK6N) Water (UNII: 059QF0KO0R) potassium citrate (UNII: EE90ONI6FF) Potassium Sorbate (UNII: 1VPU26JZZ4) Propylene Glycol (UNII: 6DC9Q167V3) propylparaben (UNII: Z8IX2SC1OH) Citric Acid Monohydrate (UNII: 2968PHW8QP) Aspartame (UNII: Z0H242BBR1) methylparaben (UNII: A2I8C7HI9T) D&C Red No. 33 (UNII: 9DBA0SBB0L) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:45737-207-16 473 mL in 1 BOTTLE; Type 0: Not a Combination Product 10/01/2009 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part341 10/01/2009 Labeler - Advanced Generic Corporation (831762971)