EQUALINE ACID REDUCER- cimetidine tablet
Supervalu Inc
----------
022 Drug Facts
Uses
- •
- relieves heartburn associated with acid indigestion and sour stomach
- •
- prevents heartburn associated with acid indigestion and sour stomach brought on by eating or drinking certain food and beverages
Warnings
Allergy alert: Do not use if you are allergic to cimetidine or other acid reducers
Do not use
- •
- if you have trouble or pain swallowing food, vomiting with blood, or bloody or black stools. These may be signs of a serious condition. See your doctor.
- •
- with other acid reducers
Ask a doctor before use if you have
- •
- frequent chest pain
- •
- frequent wheezing, particularly with heartburn
- •
- unexplained weight loss
- •
- nausea or vomiting
- •
- stomach pain
- •
- had heartburn over 3 months. This may be a sign of a more serious condition.
- •
- heartburn with lightheadedness, sweating or dizziness
- •
- chest pain or shoulder pain with shortness of breath; sweating; pain spreading to arms, neck or shoulders; or lightheadedness
Ask a doctor or pharmacist before use if you are taking
- •
- theophylline (oral asthma medicine)
- •
- warfarin (blood thinning medicine)
- •
- phenytoin (seizure medicine)
If you are not sure you are taking one of these medicines, talk to your doctor or pharmacist.
Directions
- •
- adults and children 12 years and over:
- •
- to relieve symptoms, swallow 1 tablet with a glass of water
- •
- to prevent symptoms, swallow 1 tablet with a glass of water right before or any time up to 30 minutes before eating food or drinking beverages that cause heartburn
- •
- do not take more than 2 tablets in 24 hours
- •
- children under 12 years: ask a doctor
Other information
- •
- do not use if printed foil under cap is broken or missing
- •
- store at 20°-25°C (68°-77°F)
Inactive ingredients
hypromellose, magnesium stearate, microcrystalline cellulose, polyethylene glycol, polysorbate 80, povidone, pregelatinized starch, sodium lauryl sulfate, sodium starch glycolate, titanium dioxide
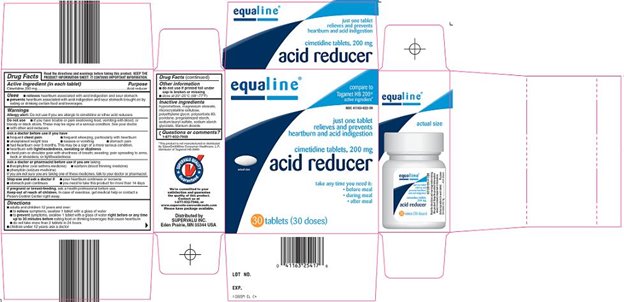
Principal Display Panel
Compare to Tagamet HB 200® active ingredient
Just One Tablet Relieves and Prevents Heartburn and Acid Indigestion
Cimetidine Tablets, 200 mg
Acid Reducer
Take Any Time you need it:
Before Meal
During Meal
After Meal
Actual Size
# Doses {Replace "#" with number in net content}
Acid Reducer Carton
| EQUALINE ACID REDUCER
cimetidine tablet |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Supervalu Inc (006961411) |
Revised: 11/2017
Document Id: 8b076af1-3738-4d7f-b693-6d419eb27bf1
Set id: 26bb576e-19c9-4103-b7bd-3bc0cf286eaa
Version: 2
Effective Time: 20171126
Supervalu Inc