FIBER LAXATIVE- calcium polycarbophil tablet
Geri-Care Pharmaceutical Corp
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
gc449
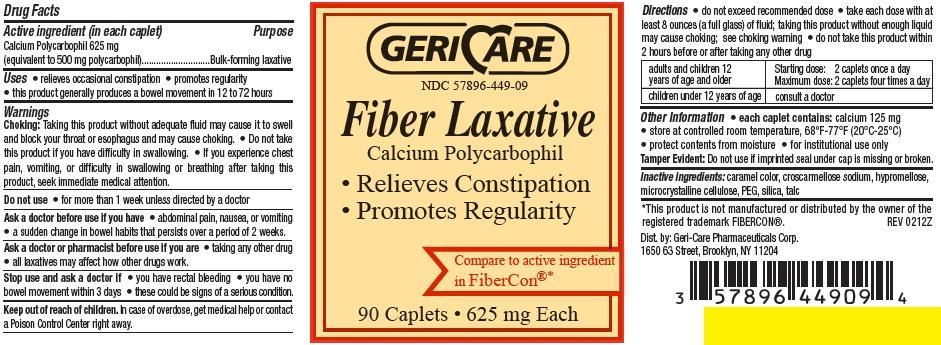
Active ingredient (in each caplet)
Calcium Polycarbophil 625 mg
(equivalent to 500 mg polycarbophil)
Uses
- relieves occasional constipation
- promotes regularity
- this product generally produces a bowel movement in 12 to 72 hours
Warnings
Choking: Taking this product without adequate fluid may cause it to swell and block your throat or esophagus and may cause choking.
• Do not take this product if you have difficulty in swallowing.
• If you experience chest pain, vomiting, or difficulty in swallowing or breathing after taking this product, seek immediate medical attention.
Do not use
• for more than 1 week unless directed by a doctor
Ask a doctor before use if you have
• abdominal pain, nausea, or vomiting
• a sudden change in bowel habits that persists over a period of 2 weeks.
Ask a doctor or pharmacist before use if you are
• taking any other drug
• all laxatives may affect how other drugs work.
Stop use and ask a doctor if
• you have rectal bleeding
• you have no bowel movement within 3 days
• these could be signs of a serious condition.
Directions
• do not exceed recommended dose
• take each dose with at least 8 ounces (a full glass) of fluid; taking this product without enough liquid may cause choking; see choking warning
• do not take this product within 2 hours before or after taking any other drug
| adults and children 12
years of age and older | Starting dose: 2 caplets once a day
Maximum dose: 2 caplets four times a day |
| Starting dose: 2 caplets once a day
Maximum dose: 2 caplets four times a day | consult a doctor
|
Other information
• each caplet contains: calcium 125 mg
• store at controlled room temperature, 68°F-77°F (20°C-25°C)
• protect contents from moisture
• for institutional use only
Tamper Evident: Do not use if imprinted seal under cap is missing or broken.
| FIBER LAXATIVE
calcium polycarbophil tablet |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Geri-Care Pharmaceutical Corp (611196254) |
| Registrant - Geri-Care Pharmaceutical Corp (611196254) |