ANTIBACTERIAL CLEAN AND SMOOTH GENTLE SKIN CLEANSER- triclosan solution
Ecolab Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
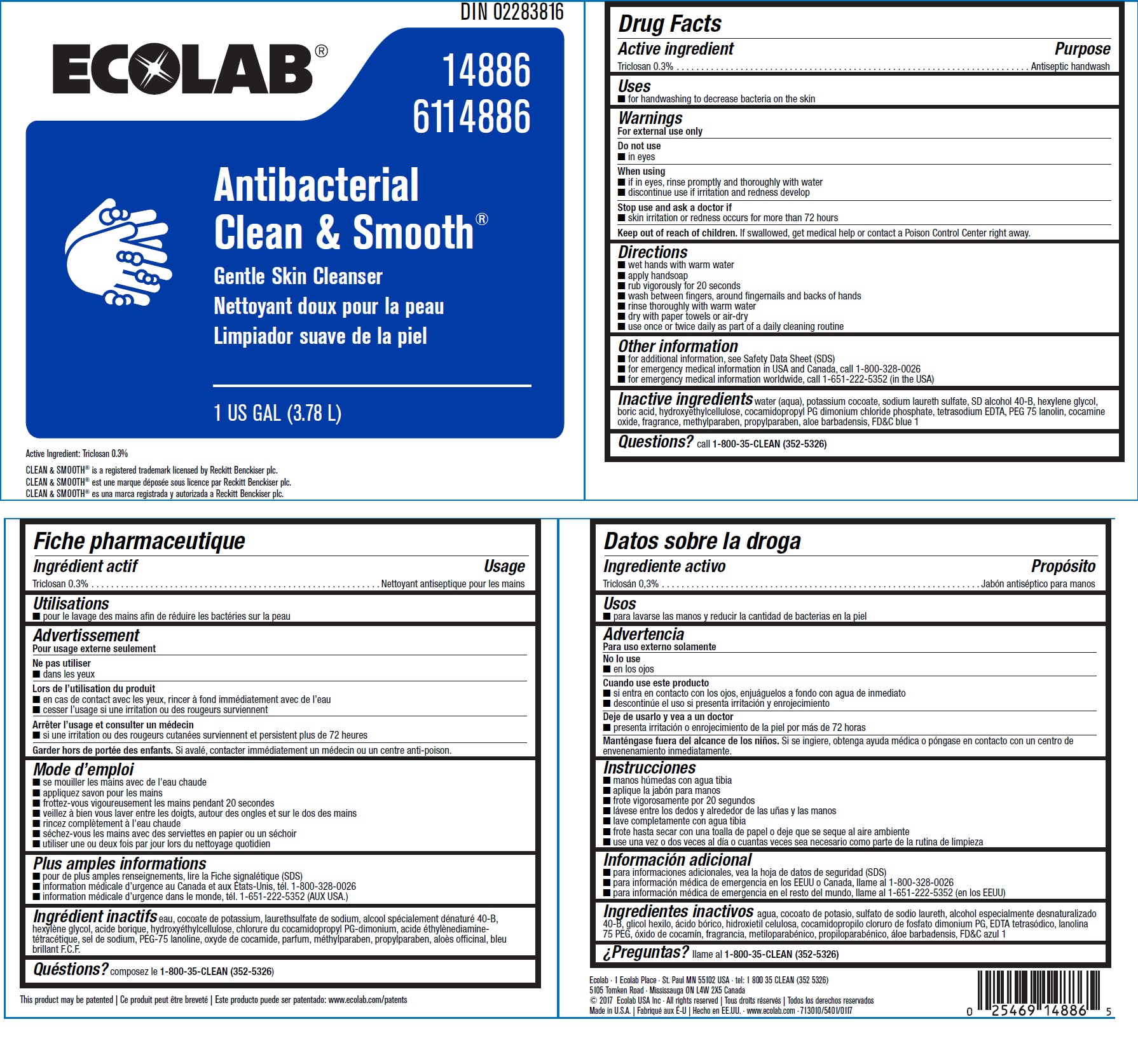
Drug Facts
Warnings
- For external use only
Directions
- Wet hands with warm water
- Apply handsoap
- Rub vigorously for 20 seconds
- Wash between fingers, around fingernails and backs of hands
- Rinse thoroughly with warm water
- Dry with paper towels or air-dry
- Use once or twice daily as part of a daily cleaning routine
Other information
- For additional information, see Safety Data Sheet (SDS)
- For emergency medical information in USA and Canada, cal 1-800-328-0026
- For emergency medical information worldwide, call 1-651-222-5352 (in the USA)
Inactive ingredients
water (aqua), potassium cocoate, sodium laureth sulfate, SD alcohol 40-B, hexylene glycol, boric acid, hydroxyethylcellulose, cocamidopropyl PG dimonium chloride phosphate, tetrasodium EDTA, PEG 75 lanolin, cocamine oxide, fragrance, methylparaben, propylparaben, aloe barbadensis, FDC blue 1
Principal Display Panel
DIN 02283816
Ecolab®
14886
6114886
Antibacterial
Clean & Smooth®
Gentle Skin Cleanser
1 U.S. GAL (3.78 L)
Active Ingredient: Triclosan 0.3%
CLEAN and SMOOTH® is a registered trademark licensed by Reckitt Benckiser plc.
Ecolab · 1 Ecolab Place · St. Paul MN 55102 USA · tel: 1 800 35 CLEAN (352 5326)
5105 Tomken Road · Mississauga ON L4W 2X5 Canada
© 2017 Ecolab USA Inc · All rights reserved
Made in U.S.A. · www.ecolab.com · 713010/5401/0117
| ANTIBACTERIAL CLEAN AND SMOOTH
GENTLE SKIN CLEANSER
triclosan solution |
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
| Labeler - Ecolab Inc. (006154611) |