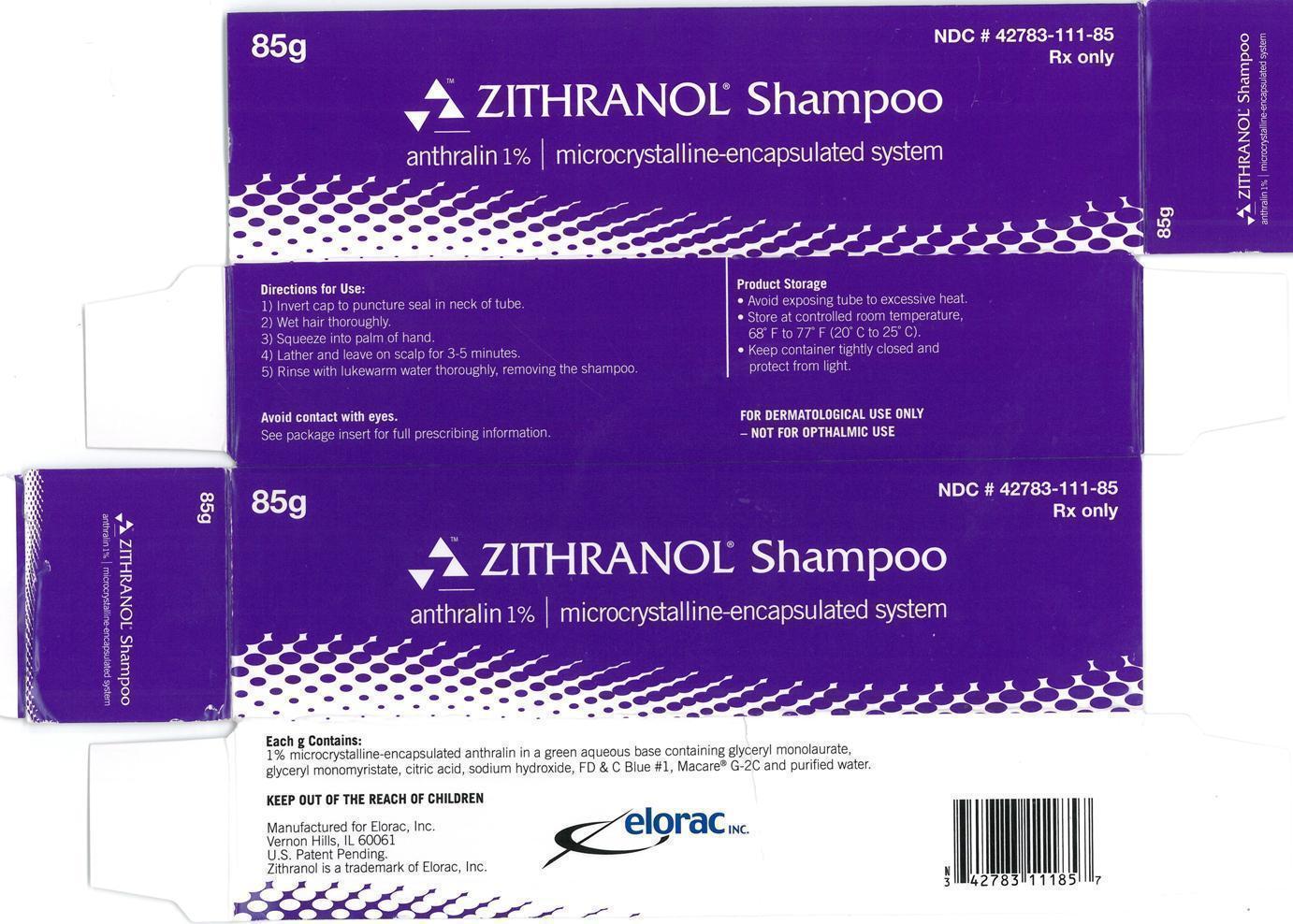
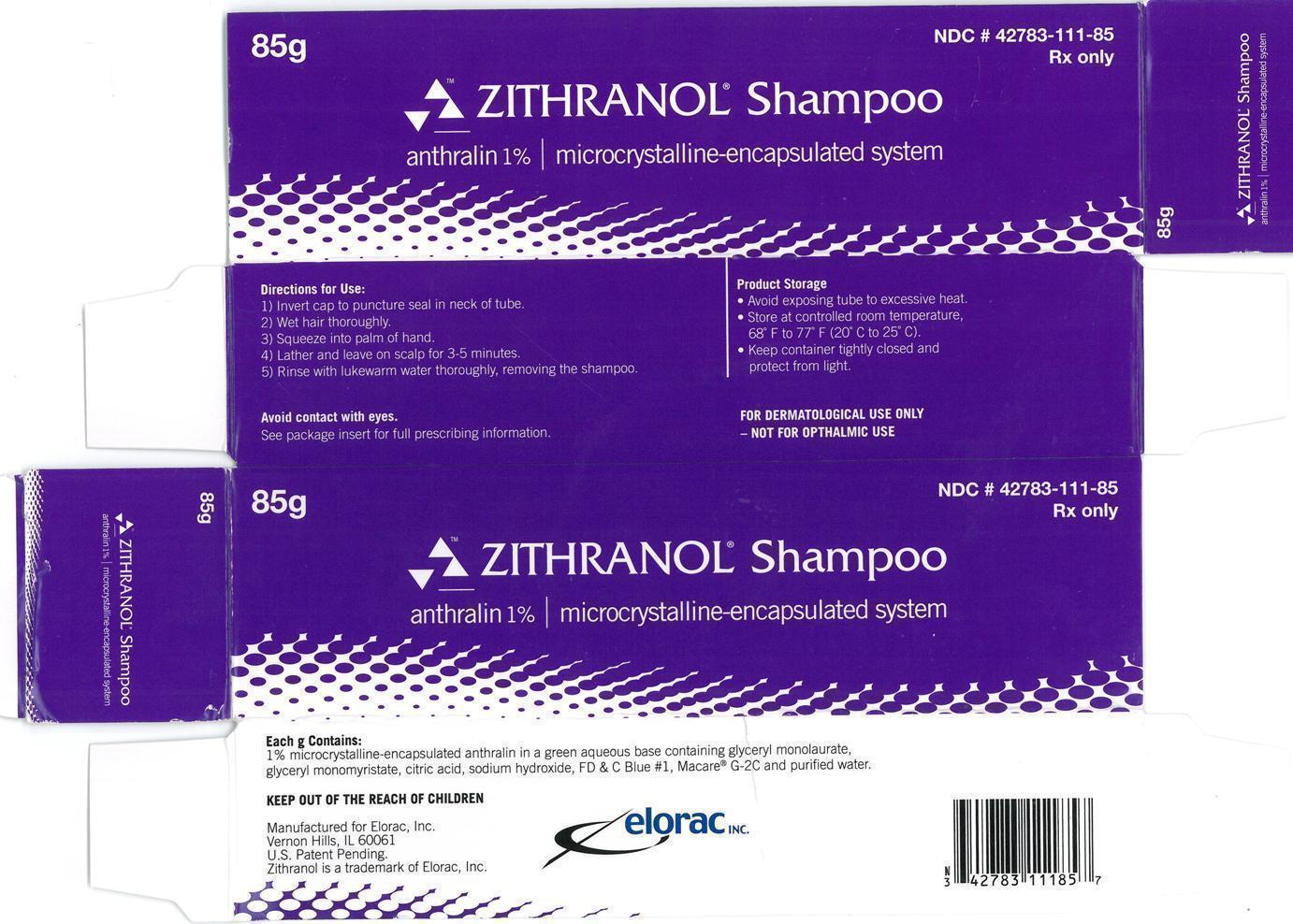
Label: ZITHRANOL- anthralin shampoo
-
Contains inactivated NDC Code(s)
NDC Code(s): 42783-111-07, 42783-111-85 - Packager: Elorac Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated October 17, 2014
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
Zithranol Shampoo anthralin 1% | microcrystalline-encapsulated system
Package Insert:
DESCRIPTION
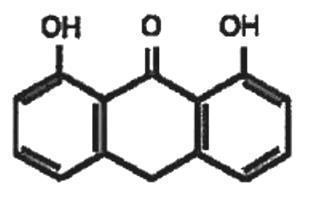
Zithranol Shampoo contains 1% anthralin in a shampoo base containing glyceryl monolaurate, glyceryl monomyristate, citric acid, sodium hydroxide, FD&C Blue No. 1, Macare® G-2C, and purified water. The chemical name of anthralin is 1,8-dihydroxy-9-anthrone and its structural formula is:
CLINICAL PHARMACOLOGY
Zithranol Shampoo contains anthralin, a synthetic compound whose precise mechanism of antipsoriatic action is not yet fully understood. However, numerous studies have demonstrated antiproliferative and antiinflammatory effects of anthralin on psoriatic and normal skin. The antiproliferative effects of anthralin appear to result from both an inhibition of DNA synthesis as well as from its strong reducing properties. Recently, anthralin’s effectiveness as an antipsoriatic agent has also been in part attributed to its ability to induce lipid peroxidation and reduce levels of endothelial adhesion molecules, which are markedly elevated in psoriatic patients. Unlike retinoids and PUVA, anthralin does not inhibit liver microsomal enzyme activity; consequently, the likelihood of adverse drug interactions is greatly reduced when other agents are administered concomitantly with anthralin.
INDICATIONS AND USAGE
Zithranol Shampoo for the treatment of scalp psoriasis in subjects 12 years of age and older (see PRECAUTIONS). Patients should be instructed to use Zithranol Shampoo for the minimum time period necessary to achieve the desired results.
CONTRAINDICATIONS
Use of Zithranol Shampoo is contraindicated in patients who are hypersensitive to anthralin or to any ingredient in this preparation.
WARNINGS
Avoid contact with the eyes or mucous membranes. Discontinue use if a sensitivity reaction occurs or if excessive irritation develops.
PRECAUTIONS
General: For external use only. Contact with fabrics, plastics and other materials may cause staining and should be avoided. Always wash hands thoroughly after use. Keep container tightly capped when not in use. Avoid excessive heat. Keep out of the reach of children.
Carcinogenesis, mutagenesis, impairment of fertility: Although long-term studies in animals have not been performed to evaluate the carcinogenic potential of the drug, there have been no reports to suggest carcinogenic effects in humans after many years of clinical use.
Pregnancy:
Teratogenic Effects: Pregnancy Category C
Animal reproduction studies have not been conducted with Zithranol Shampoo. It is also not known whether Zithranol Shampoo can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Zithranol Shampoo should be given to a pregnant woman only if clearly needed.Nursing mothers: Because many drugs are excreted in human milk, caution should be exercised when Zithranol Shampoo is administered to a nursing woman.
Pediatric use: Safety and effectiveness in children have not been established.
ADVERSE REACTIONS
Very few instances of contact allergic reactions to anthralin have been reported. Transient primary irritation and staining of hair or skin may occur. The temporary discoloration of hair and skin may be minimized by careful application. Staining of fabrics may be permanent so contact should be avoided.
DOSAGE AND ADMINISTRATION
Zithranol Shampoo should be applied onto wet scalp 3 to 4 times per week (or as directed by the physician). Lather and leave on scalp for 3 to 5 minutes and then rinse thoroughly. Avoid contact with eyes. Although no additional shampoo is necessary to cleanse the hair, a non-medicated shampoo or conditioner can be applied following the use of Zithranol Shampoo, if desired. If no improvement is seen after 4 weeks of treatment, consult the physician.
HOW SUPPLIED
Zithranol Shampoo (anthralin 1%) microcrystalline-encapsulated system is supplied in tubes.
85g NDC 42783-111-85
7.5g NDC 42783-111-07
(physician sample size)Store at controlled room temperature 68°F to 77°F (20°C to 25°C).
Manufactured for:
Elorac, Inc.
Vernon Hills, IL 60061U.S. Patent Pending
Caution: Federal law prohibits dispensing without prescription.
© 2011 Elorac, Inc.
Rev. 01 (10/2011)TUBE AND CARTON PHOTO:

- Zithranol Shampoo anthralin 1% | microcrystalline encapsulated system 85g Carton Label:
- Zithranol Shampoo anthralin 1% | microcrystalline encapsulated system 85g Container Label:
- Zithranol Shampoo anthralin 1% | microcrystalline-encapsulated system 7.5g Physician Sample Container Label:
-
INGREDIENTS AND APPEARANCE
ZITHRANOL
anthralin shampooProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:42783-111 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Anthralin (UNII: U8CJK0JH5M) (Anthralin - UNII:U8CJK0JH5M) Anthralin 1 g in 100 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) SODIUM HYDROXIDE (UNII: 55X04QC32I) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) GLYCERYL LAURATE (UNII: Y98611C087) GLYCERYL MYRISTATE (UNII: 6992Y81827) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) GLYCERETH-2 COCOATE (UNII: JWM00VS7HC) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:42783-111-85 12 in 1 CASE 1 1 in 1 CARTON 1 85 g in 1 TUBE 2 NDC:42783-111-07 24 in 1 CASE 2 1 in 1 CARTON 2 7.5 g in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 03/05/2012 Labeler - Elorac Inc. (832590009) Registrant - Elorac, Inc. (832590009)