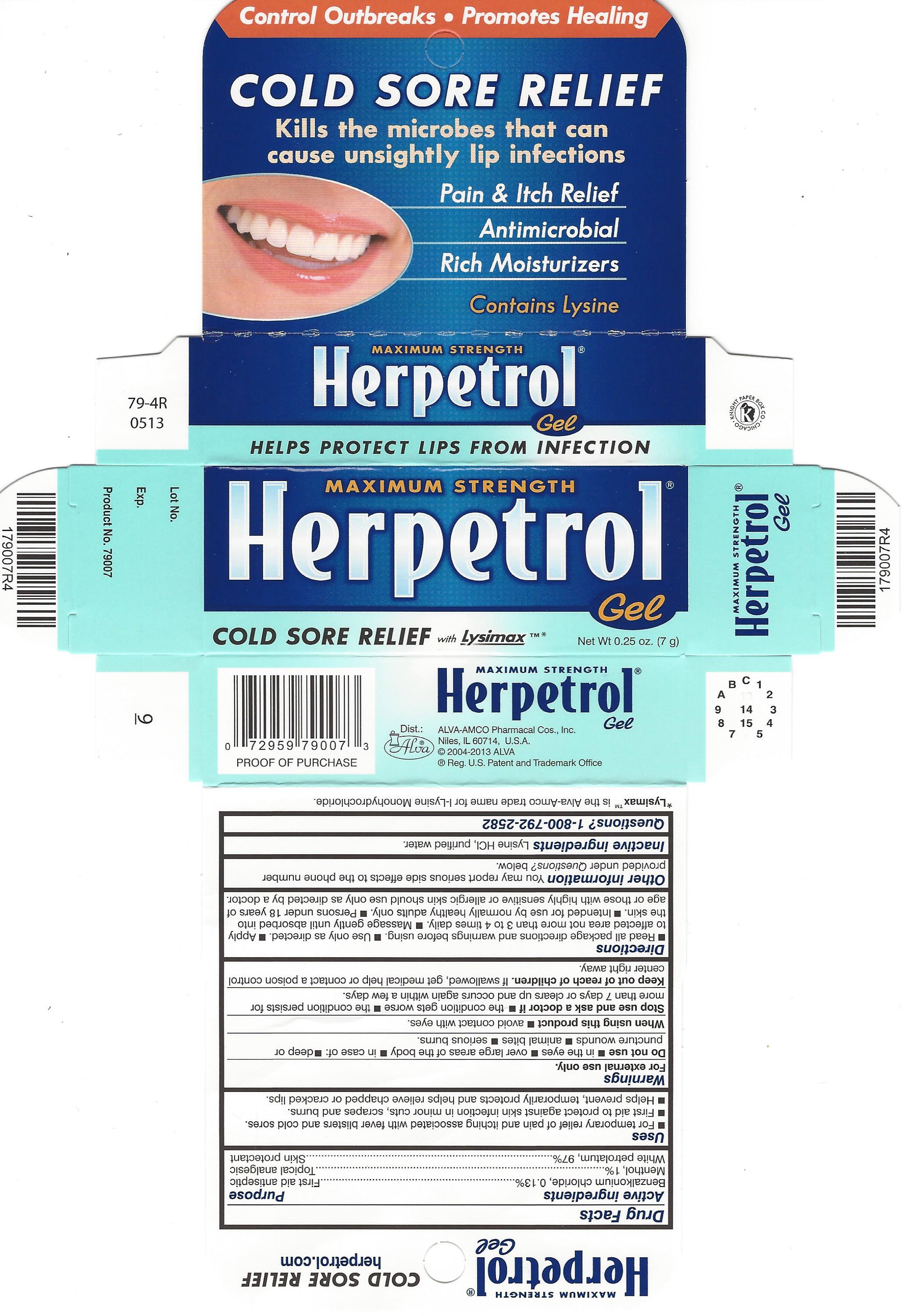
HERPETROL- benzalkonium chloride, menthol, petrolatum ointment
Alva-Amco Pharmacal Companies, Inc.
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
Herpetrol Gel
Uses
- For temporary relief of pain and itching associated with fever blisters and cold sores.
- First aid to protect against skin infection in minor cuts, scrapes and burns.
- Helps prevent, temporarily protects and helps relieve chapped or cracked lips.
Do not use
- in the eyes
- over large areas of the body
- in case of
-
- deep or puncture wounds
- animal bites
- serious burns
Stop use and ask a doctor if
- the condition gets worse
- persists for more than 7 days or clears up and occurs again within a few days.
Keep out of reach of children. If swallowed, get medical help or contact a poison control center right away.
Directions
- Read all package directions and warnings before using.
- Use only as directed.
- Apply to affected area not more than 3 to 4 times daily.
- Massage gently until absorbed into the skin.
- Intended for use by normally healthy adults only.
- Persons under 18 years of age or those with highly sensitive or allergic skin should use only as directed by a doctor.
| HERPETROL
benzalkonium chloride, menthol, petrolatum ointment |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Alva-Amco Pharmacal Companies, Inc. (042074856) |
Revised: 12/2018
Document Id: 7cee5b84-c467-6bb2-e053-2a91aa0a5676
Set id: 2504eae6-68eb-4879-a2e6-dd8a926abcce
Version: 3
Effective Time: 20181213
Alva-Amco Pharmacal Companies, Inc.