RES-Q ANALGESIC TOPICAL PAIN RELIEF- lidocaine hydrochloride liquid
CONAIR CORPORATION
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
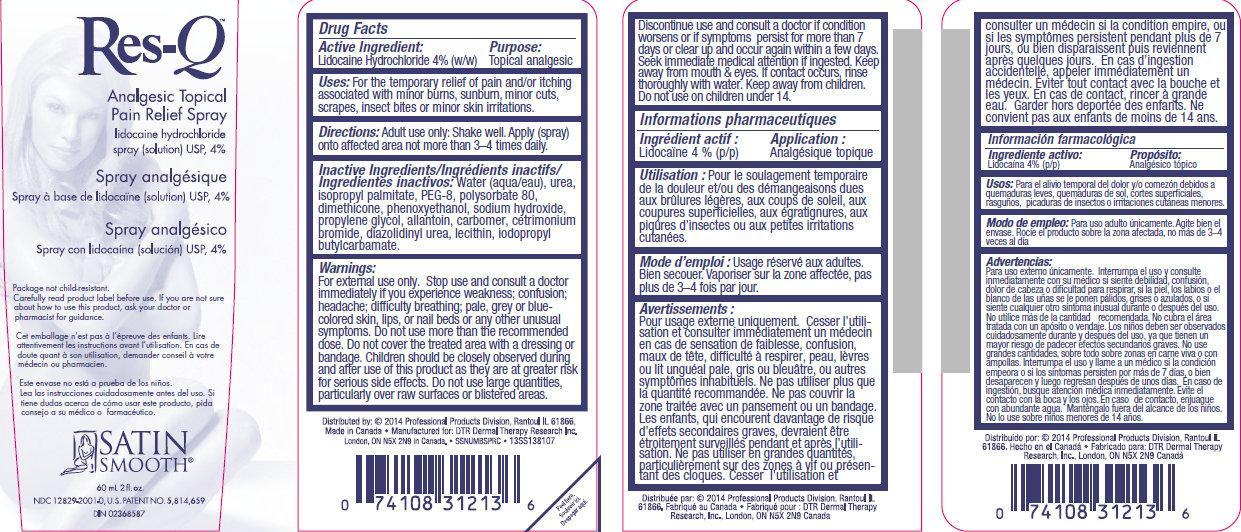
Res-Q Analgesic Topical Pain Relief Spray
Uses:
For the temporary relief of pain and/or itching associated with minor burns, sunburn, minor cuts, scrapes, insect bites or minor skin irritations.
Directions:
Adult use only: Shake well. Apply (spray) onto affected area not more than 3-4 times daily.
Inactive Ingredients:
Water, urea, isopropyl palmitate, PEG-8, Polysorbate 80, dimethicone, phenoxyethanol, sodium hydroxide, propylene glycol, allantoin, carbomer, cetrimonium bromide, diazolidinyl urea, lecithin, iodopropyl butylcarbamate.
Warnings
For external use only.
Stop use and consult a doctor immediately if you experience weakness; confusion; headache; difficulty breathing; pale, grey or blue-colored skin, lips, or nail beds or any other unusual symptoms.
Do not use more than the recommended dose.
Do not cover the treated area with a dressing or bandage.
Children should be closely observed during and after use of this product as they are at greater risk for serious side effects.
Do not use large quantities, particularly over raw surfaces or blistered areas.
Discontinue use and consult a doctor if condition worsens or if symptoms persist for more than 7 days or clear up and occur again within a few days.
Seek immediate medical attention if ingested.
Keep away from mouth and eyes.
If contact occurs, rinse thoroughly with water.
| RES-Q ANALGESIC TOPICAL PAIN RELIEF
lidocaine hydrochloride liquid |
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
| Labeler - CONAIR CORPORATION (001661222) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| CSR Cosmetic Solutions Inc. | 243501959 | manufacture(12829-2001) | |