TUSSIN DM SUGAR FREE NON DROWSY- dextromethorphan hbr, guaifenesin liquid
QUALITY CHOICE (Chain Drug Marketing Association)
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
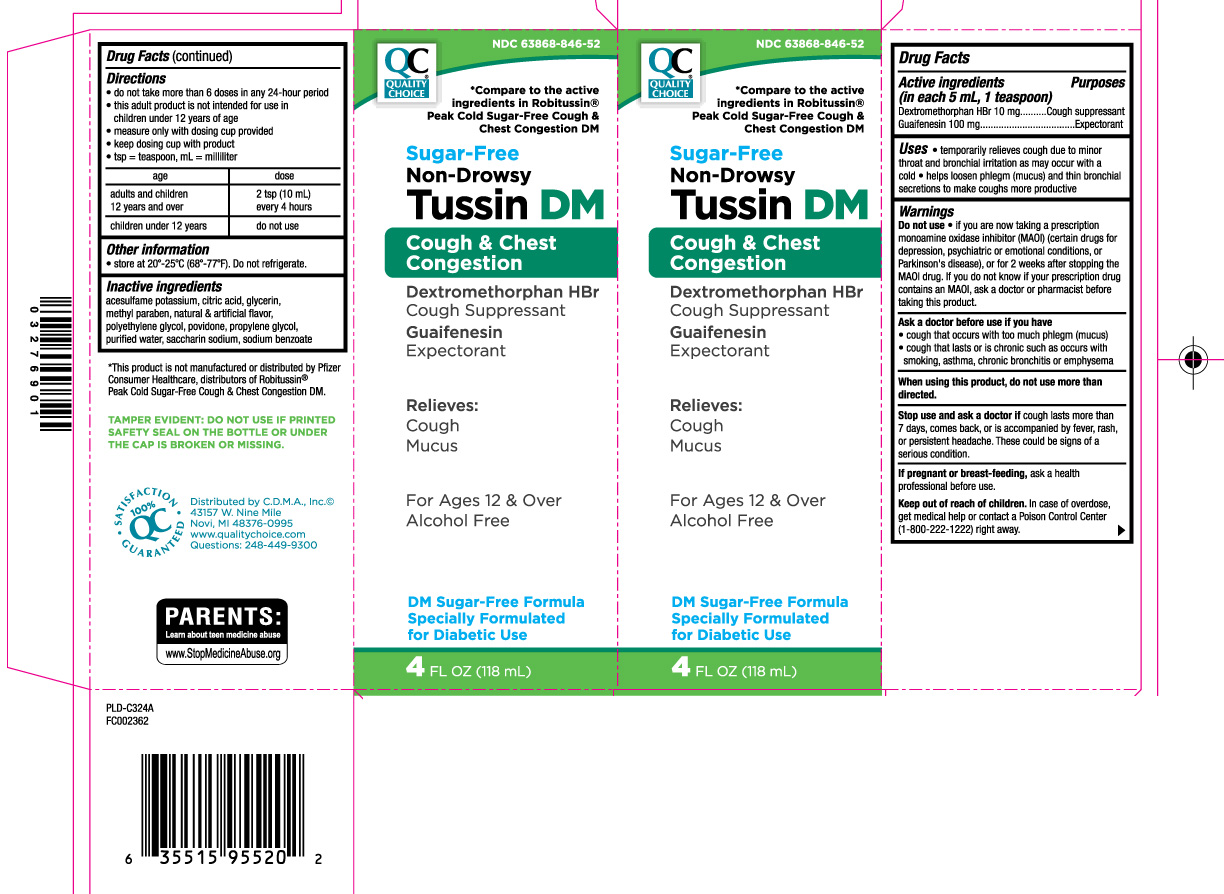
Drug Facts
Uses
- temporarily relieves cough due to minor throat and bronchial irritation as may occur with a cold
- helps loosen phlegm (mucus) and thin bronchial secretions to make coughs more productive
Warnings
Do not use
- if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson’s disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have
- cough that occurs with too much phlegm (mucus)
- cough that lasts or is chronic such as occurs with smoking, asthma, chronic bronchitis or emphysema
Directions
- do not take more than 6 doses in any 24-hour period
- this adult product is not intended for use in children under 12 years of age
- measure only with dosing cup provided
- keep dosing cup with product
- tsp = teaspoon, mL = milliliter
| age | dose |
| adults and children 12 years and over | 2 tsp (10 mL) every 4 hours |
| children under 12 years | do not use |
Inactive ingredients
acesulfame potassium, citric acid, glycerin, methyl paraben, natural & artificial flavor, polyethylene glycol, povidone, propylene glycol, purified water, saccharin sodium, sodium benzoate
Principal Display Panel
*Compare to the active ingredients in Robitussin® Peak Cold Sugar-Free Cough & Chest Congestion DM
Sugar-Free
Non-Drowsy
Tussin DM
Cough & Chest Congestion
Dextromethorphan HBr Cough Suppressant
Guaifenesin Expectorant
Relieves:
Cough
Mucus
For Ages 12 & Over
Alcohol Free
DM Sugar-Free Formula Specially Formulated for Diabetic Use
FL OZ (mL)
*This product is not manufactured or distributed by Pfizer Consumer Healthcare, distributors of Robitussin® Peak Cold Sugar-Free Cough & Chest Congestion DM.
TAMPER EVIDENT: DO NOT USE IF PRINTED SAFETY SEAL ON THE BOTTLE OR UNDER THE CAP IS BROKEN OR MISSING.
Distributed by C.D.M.A., Inc.
43157 W. Nine Mile
Novi, MI 48376-0995
www.qualitychoice.com
Questions: 248-449-9300
| TUSSIN DM
SUGAR FREE NON DROWSY
dextromethorphan hbr, guaifenesin liquid |
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Labeler - QUALITY CHOICE (Chain Drug Marketing Association) (011920774) |