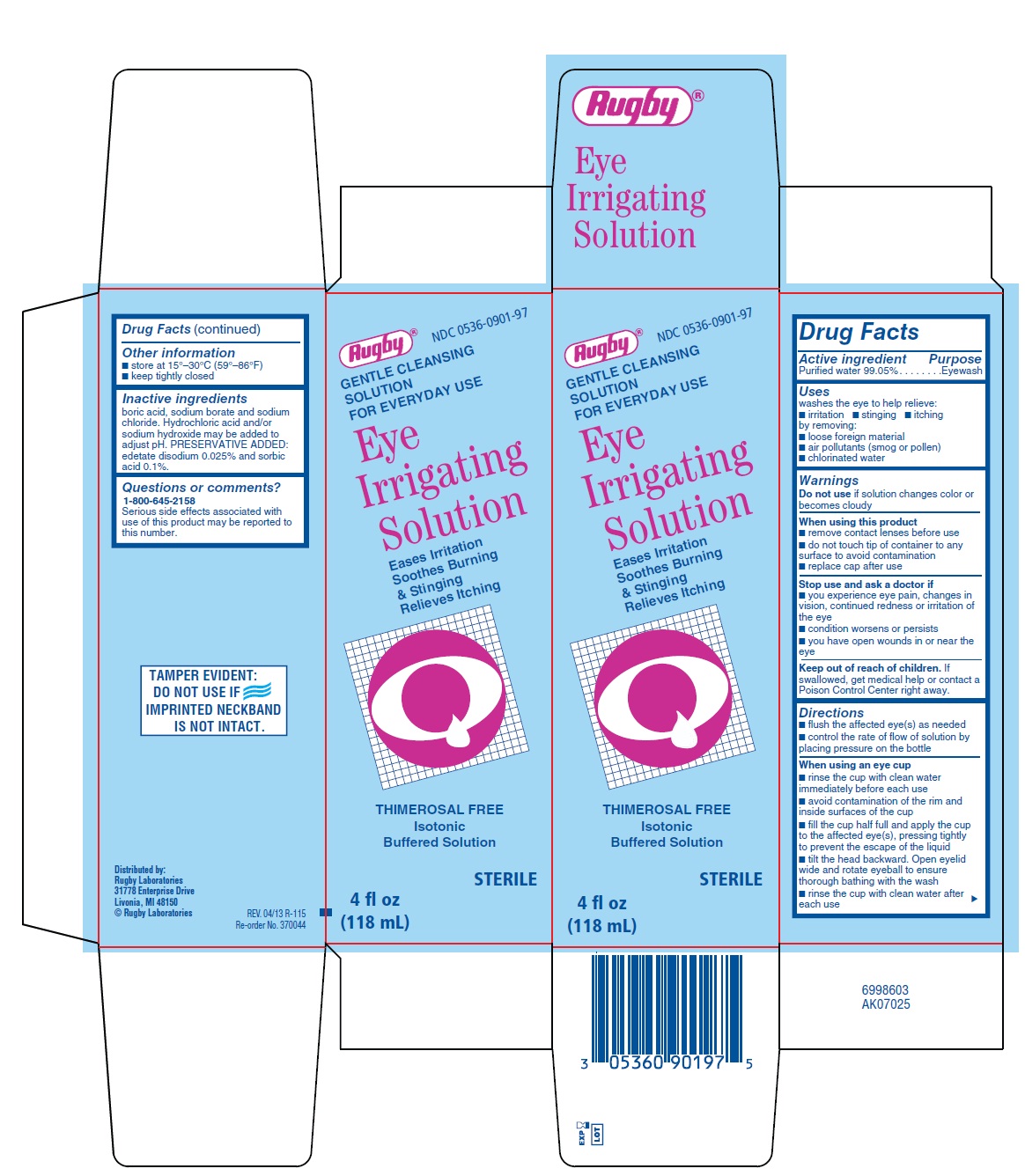
EYE IRRIGATING- purified water solution
Rugby Laboratories Inc.
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
Drug Facts
Uses
washes the eye to help relieve:
- •
- irritation
- •
- stinging
- •
- itching
by removing: - •
- loose foreign material
- •
- air pollutants (smog or pollen)
- •
- chlorinated water
Warnings
Do not use if solution changes color or becomes cloudy
When using this product
- •
- remove contact lenses before use
- •
- do not touch tip of container to any surface to avoid contamination
- •
- replace cap after use
Stop use and ask a doctor if
- •
- you experience eye pain, changes in vision, continued redness or irritation of the eye
- •
- condition worsens or persists
- •
- you have open wounds in or near the eye
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- •
- flush the affected eye(s) as needed
- •
- control the rate of flow of solution by placing pressure on the bottle
When using an eye cup
- •
- rinse the cup with clean water immediately before each use
- •
- avoid contamination of the rim and inside surfaces of the cup
- •
- fill the cup half full and apply the cup to the affected eye(s), pressing tightly to prevent the escape of the liquid
- •
- tilt the head backward. Open eyelid wide and rotate eyeball to ensure thorough bathing with the wash
- •
- rinse the cup with clean water after each use
Inactive ingredients
boric acid, sodium borate and sodium chloride. Hydrochloric acid and/or sodium hydroxide may be added to adjust pH. PRESERVATIVE ADDED: edetate disodium 0.025% and sorbic acid 0.1%.
| EYE IRRIGATING
purified water solution |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Rugby Laboratories Inc. (079246066) |
Revised: 9/2019
Document Id: 420fdca4-e125-453c-845d-838b57c7bd24
Set id: 230a9605-f7a6-4429-a358-04f112101379
Version: 3
Effective Time: 20190905
Rugby Laboratories Inc.