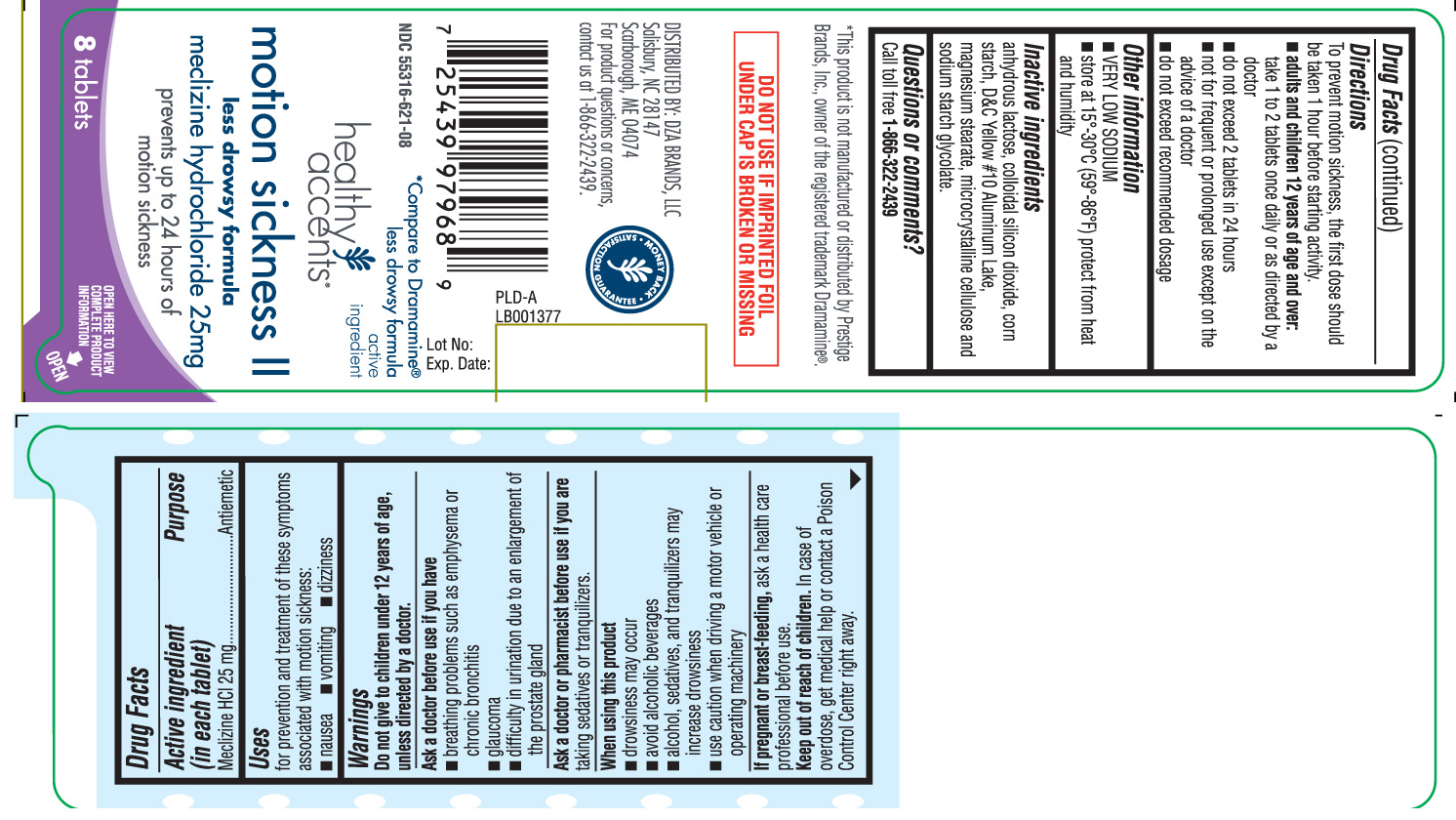
MOTION SICKNESS II- meclizine hcl tablet
Healthy Accents (DZA Brands, LLC)
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Drug Facts
Uses
for prevention and treatment of these symptoms associated with motion sickness:
- nausea
- vomiting
- dizziness
Warnings
Do not give to children under 12 years of age, unless directed by a doctor.Ask a doctor before use if you have
- breathing problem such as emphysema or chronic bronchitis
- glaucoma
- difficulty in urination due to an enlargement of the prostate gland
Directions
To prevent motion sickness, the first dose should be taken 1 hour before starting activity.
- adults and children 12 years of age and over: take 1 to 2 tablets once daily or as directed by a doctor
- do not exceed 2 tablets in 24 hours
- not for frequent or prolonged use except on the advice of a doctor
- do not exceed recommended dosage
Inactive ingredients
anhydrous lactose, colloidal silicon dioxide, corn starch, D&C Yellow# 10 Aluminium Lake, magnesium stearate, microcrystalline cellulose and sodium starch glycolate.
Principal Display Panel
*Compare to Dramamine® Less Drowsy Formula active ingredient
Motion Sickness II
Less Drowsy Formula
Meclizine Hydrochloride 25 mg
prevents up to 24 hours of motion sickness
Tablets
*This product is not manufactured or distributed by Prestige Brands, Inc., owner of the registered trademark Dramamine®.
DO NOT USE IF IMPRINTED FOIL UNDER CAP IS BROKEN OR MISSING
DISTRIBUTED BY: DZA BRANDS, LLC
Salisbury, NC 28147
Scarborough, ME 04074
| MOTION SICKNESS II
meclizine hcl tablet |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Healthy Accents (DZA Brands, LLC) (090322194) |